Learning Objectives
After completing this application-based continuing education activity, pharmacists and pharmacy technicians will be able to
| · Identify patient populations in which ketamine use is justified based on its FDA approved indications and for off-labeled use where it has been sufficiently studied |
| · Compare the different formulations of ketamine and its “kissing cousins” |
| · Describe potential risks associated with ketamine use |
Release Date:
Release Date: October 27, 2025
Expiration Date: October 27, 2028
Course Fee
Pharmacists: $7
Pharmacy Technicians: $4
There is no grant funding for this CE activity
ACPE UANs
Pharmacist: 0009-0000-25-071-H08-P
Pharmacy Technician: 0009-0000-25-071-H08-T
Session Codes
Pharmacist: 22YC62-FXK22
Pharmacy Technician: 22YC62-KXT46
Accreditation Hours
2.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-25-071-H08-P/T will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Alexis Hicks, PharmD.
CVS Health
West Hartford, CT
Canyon Hopkins, PharmD.
Medical Professional Ethos Cannabis
Pittsburgh, PA
Alexis Redfield, PharmD.
CVS
Vernon, CT
Ashley Walsh, PharmD.
Mohegan Pharmacy
Uncasville, CT
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Drs. Hicks, Hopkins, Redfield, and Walsh do not have any relationships with ineligible companies and therefore have nothing to disclose.
ABSTRACT
Ketamine is Food and Drug Administration-approved as a general anesthetic. Researchers found higher dose ketamine therapy had a more desirable adverse effect profile than the previously used anesthetic phencyclidine (PCP). N-methyl-D-aspartate (NMDA) antagonism from subanesthetic ketamine doses produces dissociative and analgesic effects. As such, prescribers are exploring off-label uses for ketamine in patients with agitation, depression, and pain while considering potential risks to multiple organ systems. Ketamine has the potential to cause complications and providers need to monitor patients closely. Illicit and inappropriate use by abusers and untrained law enforcement officers highlight ketamine’s potentially harmful effects. Educating patients and healthcare providers is vital to allow potential benefits while minimizing harm.
CONTENT
Content
Introduction
Consider this: It’s 10:30 PM on a Friday night, 30 minutes before you leave for the weekend. Suddenly, from across the emergency department you hear, “Get OFF me! No, I have not t-t-taken anything! If you come ANY CLOSER, things are going to get physical!” Not a moment later, an order pops up for ketamine hydrochloride 500 mg intramuscularly (IM) for severe agitation. Concerned, a colleague asks you, “Is this safe? Is this effective? I have never seen a dose this high before. Isn’t this just for horses?”
Ketamine made its debut in human clinical practice in the 1960s when several chemists at Parke Davis Company were searching for an anesthetic with similar effects to phencyclidine (PCP). PCP, ketamine’s notorious kissing cousin, was a promising new anesthetic in the 1950s because of its dissociative effects. However, the chemists quickly became unimpressed with its adverse effect profile (i.e., long-lasting psychoactive effects after anesthesia). Humans experienced intense prolonged emergence delirium following PCP anesthesia, relegating its use to veterinary practice.1 Chemists searched for a better anesthetic and found ketamine, which has similar dissociative effects without PCP’s negative consequences. Ketamine is a more desirable anesthetic because it has a shorter half-life (2.5 hours) compared to PCP (21 hours) and it causes less delirium.1,2
Prescribers have begun using ketamine for several off-label uses and patients have also started using the drug or structural analogs in a variety of formulations illicitly. Pharmacists and technicians can ensure ketamine’s safe use by keeping current with new formulations and indications, both approved and unapproved. This continuing education activity will dive into the clinical and social consequences of ketamine use.
What’s Ketamine?
Ketamine is a schedule III-controlled substance approved by the U.S. Food and Drug Administration (FDA) for use as a general anesthetic for diagnostic and surgical procedures.2 Ketamine is commercially available in the United States as a solution/injection under its brand (Ketalar) and as generic ketamine.2 Healthcare providers most often use intravenous (IV) ketamine, but it may be used IM or compounded into an oral solution.
Healthcare providers also use ketamine off-label for analgesia, agitation, and major depressive disorder. These indications emanate from ketamine’s mechanism of action: it acts specifically on the N-methyl-D-Aspartate (NMDA) receptor as a non-competitive antagonist to block glutamate binding.3 Glutamate, a major excitatory neurotransmitter, binds to receptors throughout the nervous system. The NMDA receptor is an ionotropic receptor responsible for the brain’s neuroplasticity, memory, learning, and recovery.4-6 Blocking this receptor with high ketamine doses (ranging from 0.5 to 2 mg/kg) results in dissociation, decreases in spinal reflexes, and produces a cataleptic state (loss of voluntary movements and reduced consciousness) that is applicable to its current clinical use in anesthesia.2 However, at low doses, ketamine can produce analgesia and stimulate new pathways within the brain that reduce depressive symptoms and improve mood.
Although ketamine has useful applications in medicine, prescribers must be aware of the adverse effects and risk factors associated with use and should consider how these effects apply to their patients before initiating the medication. Ketamine adversely impacts multiple organ systems (see Table 1), including but not limited to the cardiovascular system. Increases in blood pressure and heart rate are important cardiovascular effects associated with ketamine therapy.2 These cardiovascular effects make it a drug of choice for anesthesia induction in patients with cardiovascular shock, where it anesthetizes patients while improving blood pressure and improving organ perfusion. However, clinicians must avoid ketamine use in patients with preexisting hypertensive conditions or other patients who have limited baroreceptor buffering capacity (baroreceptor buffering is the body’s ability to sense blood pressure) because of those same effects mentioned above.6
Table 1. General Adverse Effects of Ketamine2,6
| System | Adverse effects |
| Cardiovascular | Cardiac arrhythmias, increased blood pressure,* increased heart rate |
| Central nervous system | Prolonged emergence from anesthesia,* psychosis,* dissociation,* drug dependence, increased intracranial pressure |
| Dermatologic | Injection site irritation |
| Gastrointestinal | Nausea,* vomiting, anorexia |
| Genitourinary | Lower urinary tract dysfunction, bladder dysfunction |
| Respiratory | Laryngospasm,* respiratory depression,* apnea |
| Immunologic | Anaphylaxis |
| Other | Hypersalivation, diplopia (double vision), nystagmus (uncontrollable rapid eye movement) |
*Common or serious adverse effects of ketamine use
Further contraindications include hypersensitivity to ketamine or its components.7 The American College of Emergency Physicians (ACEP) does not recommend ketamine use in patients with schizophrenia or in children younger than three months of age. The ACEP also advises against solely using ketamine as an anesthetic in procedures involving the pharynx, larynx, and bronchial tree. This recommendation primarily applies to patients with airway instability because ketamine can cause laryngospasms.5 Table 2 lists additional considerations in special populations.
Table 2. Special Population Considerations with Ketamine2,6-8
| Special population | Concerns | Recommendation |
| Pregnancy | Crosses the placenta; may have potential risk to fetus | Avoid use; evaluate benefits vs risk |
| Breastfeeding | Compatibility and safety unknown | Avoid breastfeeding to children with respiratory risk factors |
| Pediatrics | Can be given with anticholinergics to minimize hypersalivation | Refer to pediatric dosing. Avoid in infants < 3 months of age |
| Elderly | May be sensitive to dissociative adverse effects | Refer to adult dosing |
| Kidney dysfunction | No additional concerns | Refer to dosing parameters |
| Liver dysfunction | Hepatobiliary dysfunction with recurrent use | Refer to dosing parameters; monitor LFTs with repeated ketamine use |
| LFTs = liver function tests | ||
Healthcare providers should monitor patients' vital signs closely during treatment with ketamine. Anesthesiologists and pharmacists must continuously watch patients undergoing surgical or diagnostic procedures for proper induction and maintenance of dissociative effects.2 In patients who must take repeated doses of ketamine (e.g., for chronic pain management or psychiatric disorders), healthcare providers should order liver function tests at baseline and every one to two days during treatment.2,6,9,10
Ketamine’s Kissing Cousins
As shown in Figure 1, ketamine is structurally related to many compounds. The drugs in Figure 1 antagonize the NMDA receptor and exhibit a dissociative effect.1 PCP is one of the most notoriously abused drugs. Compared with ketamine, PCP is 10 times more potent and has a longer duration of action due to its strong affinity for the NMDA receptor. Both ketamine and PCP can replicate schizophrenia’s positive, negative, and cognitive symptoms and exacerbate underlying schizophrenia. But because ketamine has lower potency and a shorter duration of action, it induces fewer severe psychiatric effects than PCP.1
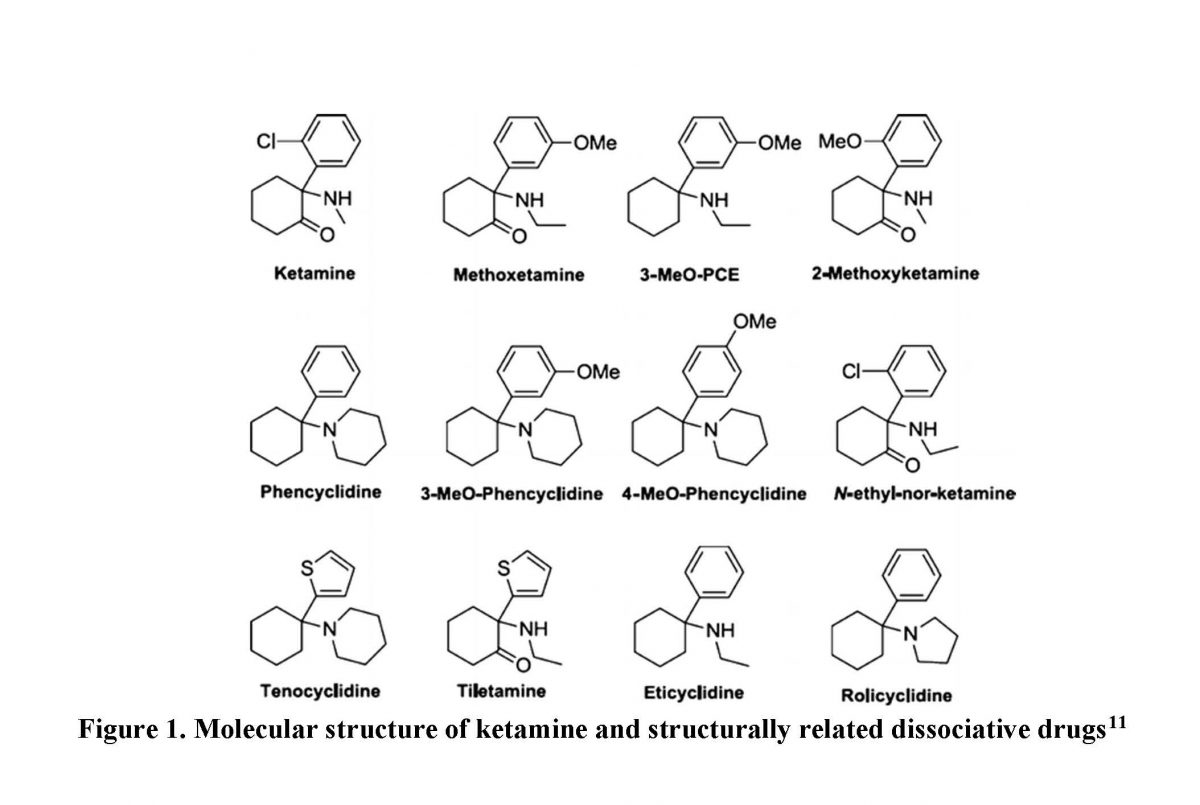
Figure 1. Molecular structure of ketamine and structurally related dissociative drugs11
Although ketamine’s labeling includes many precautions, it is an emerging option because of its therapeutic benefits. Xi Biopharmaceuticals is developing a sublingual wafer to treat acute pain while Janssen Pharmaceuticals has developed a nasal spray formulation for treatment resistant depression.12,13 Table 3 compares the current ketamine formulations that are FDA-approved or under investigation.
Table 3. Ketamine Counterparts12-14
| Cousins | Formulation | Use & Dose | Approval or Trial Phase |
| Ketalar (ketamine hydrochloride) | Injectable | Anesthesia
0.25 – 0.35 mg/kg followed by CIVI 1 mg/kg/hr |
FDA-approved |
| Wafermine (ketamine) | Sublingual Wafer | Acute Pain
25 mg, 50 mg & 75 mg PRN for 12 hrs |
End-of-Phase 2 Clinical Trials |
| Spravato (esketamine) | Nasal Spray | Treatment Resistant Depressive Disorder
28 mg, 56 mg, 84 mg twice a week |
FDA-approved |
| ABBREVIATION: CIVI = continuous intravenous infusion | |||
ABUSE, ADDICTION, DEPENDENCE
Why is Ketamine Dangerous?
Long-term ketamine abuse is associated with memory, attention, and judgment impairment. The actual risk of ketamine abuse in the general population is low compared to other substances of abuse, but patients with polysubstance abuse disorder tend to use it.15 A study examining polysubstance abuse conducted in New York City found that polydrug use occurred because of an unexpected opportunity to use ketamine after already consuming other drugs. Researchers also determined that polysubstance abusers purposefully used ketamine with another substance to achieve an individually desired effect. Oftentimes polydrug-using events occurred within a group and each member contributed something: ketamine, knowledge, other drugs, or space to use drugs.16
Currently, ketamine is only commercially available as an injectable liquid. Dealers illegally sell ketamine as a recreational injectable substance or a white powder that resembles cocaine. The Department of Justice and Drug Enforcement Administration report that illegally distributed ketamine is diverted or stolen from veterinary clinics or smuggled into the United States from Mexico.17 Dealers can then synthesize ketamine into a powder or sell it as an injectable liquid.18 Prices average from $20-$25 per dose (50 mg to 100 mg).19 Drug abusers find ketamine’s dissociative sensations and hallucinations appealing. Users can inject liquid ketamine, or snort or smoke powdered ketamine.17 Ketamine is a popular drug to facilitate physical or sexual assault because it is a colorless, tasteless, and odorless liquid making it difficult for victims to detect. Additionally, ketamine is known to cause impaired coordination, confusion, and memory loss.20
Ketamine’s IV administration started in the early 1990s. Injection events occur most frequently in large cities with high rates of homelessness, like New York City and Los Angeles.18 Researchers conducted a study with 213 people who abused IV ketamine.18 Among these users, 84% admitted to abusing ‘harder’ drugs first, with heroin predominating. Users reported their first ketamine injection happening among a group of people. This group often included people well known to them who provided knowledge and the materials for injecting.18
What attracts people to a dissociative drug with unknown psychoactive effects? Exactly that: the unknown. With most abused drugs, the user understands the effects they will experience. When someone takes ketamine, the reaction to each dose is unknown. Some users seek variety. Ketamine users have described an out of body experience that expands internal and external realms and realities.18 On the other hand, abusers also describe a “K-Hole”—an experience that they describe as near-death that results when they ingest too much ketamine.17
Timothy Wyllie, a spiritualist, describes ketamine doses as a curve over time through realms. He describes the domains abusers experience as they dose ketamine21:
- The realm “I,” for internal reality, occurs at doses 30 to 75 mg roughly 10 minutes after injection.
- The extraterrestrial reality realm, “They,” occurs at doses 75 to 150 mg approximately 15 minutes after injection.
- The realm “We,” for network creation realm, occurs at doses from 150 to 300 mg mg approximately 15 minutes after injection.
- An unknown realm exists at doses of more than 300 mg.
The doses studied for depression fall in the realm of internal reality. At these doses, users can see areas needing self-improvement that they were unaware they had the ability to fix. Drug users prefer subanesthetic doses but those that are higher than doses studied for treating depression. As the dose increases, users become so far removed from reality that “extraterrestrial” experiences begin.21
KETAMINE USES
Anesthesia
Patients unable to maintain and protect their airways require endotracheal intubation. Healthcare providers use ketamine as a sedative to facilitate rapid sequence induction and intubation (RSII), by inducing an anesthetized state, prior to paralyzing the patient. The decision to intubate is based on the patient’s Glasgow Coma Score.22,23 A score of 8 or less qualifies a patient to receive endotracheal intubation.23 Healthcare providers follow a RSII strict algorithm, shown in Table 4, detailing the order in which medications should be administered based upon the onset and duration of action.
Table 4. Algorithm of Rapid Sequence Induction & Intubation22,23
| Step of RSII | What and Why | Medications Used |
| Premedication* | Airway manipulation causes a sympathetic activation due to a pressor response. This sympathetic response leads to an increase in intracranial pressure and mean arterial pressure. | alfentanil, fentanyl, lidocaine, sufentanil |
| Sedation | Used to induce an anesthetic state before a paralytic is used and the airway manipulated. Crucial that a patient is properly sedated before paralyzed. | Also known as induction agents: etomidate, ketamine, midazolam, propofol |
| Paralytics± | Neuromuscular blocking agents are given to relax pharyngeal and diaphragmatic muscles allowing for an endotracheal tube to be placed. | rocuronium, succinylcholine, vecuronium |
*: Based upon time constraints/needs this step may be omitted
±: It is imperative to confirm a patient is properly sedated before beginning paralysis because if the patient is awake, they may feel the tube insertion
The drugs used in RSII possess unique characteristics, including IV use, quick onset, and short duration of action.23 Traditionally, etomidate has been the gold standard for RSII, but ketamine is quickly becoming a commonly used alternative.23 Table 5 highlights the differences between etomidate and ketamine.
Table 5. Comparison of Etomidate and Ketamine22,23
| Etomidate | Ketamine | |
| Dose for Induction | 0.3 mg/kg | 1.5 mg/kg or 0.1-0.5 mg/kg/min with 10% given as induction bolus |
| Onset of Action | 10-15 seconds | < 30 seconds |
| Duration of Action | 4-10 minutes | 10-15 minutes |
| Benefits | Stable hemodynamic profile, decreases metabolic rate, decreases cerebral blood flow, increases generalized seizure threshold | Sedative and analgesic properties,* cardiovascular and respiratory stimulation, and smooth muscle relaxation (beneficial in reactive airway disease, hypotensive, volume depleted, and septic patients) |
| Risks | Adrenal suppression, do not use in septic shock, lowers focal seizure threshold, increased incidence of ARDS | Potentiates effect of epinephrine, increases cardiac oxygen demand, may increase ICP,** emergent reactions, infusion related respiratory depression, hypersalivation |
| ABBREVIATIONS: ARDS = acute respiratory distress syndrome, ICP = intracranial pressure
* Ketamine can be used as a combined premedication and induction step ** Data is conflicting, however, may not be suitable for patients with head trauma |
||
The differences between etomidate and ketamine create a significant role in RSII for both drugs, but for different presenting conditions. Ketamine is gaining popularity for its use in septic patients, hypotensive patients, and those with reactive airway diseases. Choosing etomidate is preferable for patients with a hemodynamically stable profile and patients with traumatic brain injury where it could be cerebroprotective.
Analgesia (pain)
Ketamine’s use in pain management is controversial due to limited data, but this dataset is growing.15,24 Before considering subanesthetic ketamine doses, prescribers should collaborate with patients and other clinical team members to try other approved pain regimens.25 Using ketamine for its analgesic properties should be based on patient-specific criteria. The prescriber must assess the patient’s treatment goals, current medical conditions, pain types, and available protocols.
Ketamine is not discussed in available pain guidelines. Some literature recommends its use after unsuccessful trials of at least two opioids. Data supporting ketamine’s use in both acute and chronic pain management is mixed in its findings.26,27 Most trials conclude ketamine can reduce acute pain exacerbations but note that prescribers must be cautious of its adverse effects.15 Data from small trials indicate using ketamine to overcome opioid withdrawal and opioid-induced-hyperalgesia (neuropathic pain) may be possible. Ketamine has a unique ability to counteract the unfavorable responses patients might experience on chronic high-dose opioids by its mechanism of action.24,28 Overstimulated opioid receptors from high dose opioid use causes more hyperalgesia. Several small case reports describe patients on high-dose chronic opioid therapy who reduced their opioid doses after low-dose ketamine administration.29
An open labeled audit determined that IV ‘burst’ ketamine therapy improved analgesia in neuropathic pain and painful bone metastases. Researchers enrolled 39 cancer patients who were refractory to opioid therapy. Patients received bursts of low-dose ketamine (100 to 500 mg/day) over three to five days and reported somatic and neuropathic pain relief for up to eight weeks.15
Limited evidence supports oral ketamine’s effect in chronic pain and most studies that examine its use are case reports or non-comparative trials. Compared to IV administration, lower oral ketamine concentrations are associated with analgesic effects. Oral ketamine has been associated with higher serum levels of its metabolite, norketamine. This metabolite seems to contribute to oral ketamine’s analgesic effects due to its shorter half-life and ability to reach much higher peak plasma concentrations than after IV administration. However, researchers have not extensively explored this in current literature.29
Healthcare providers and patients face many hurdles when using ketamine for pain relief. Prescribers should avoid high ketamine doses that may cause a range of serious adverse effects. Unlike opioids, ketamine has a ceiling effect and maximum dose. Oral ketamine administration has a low bioavailability and is directly linked with a high rate of adverse effects.15,27
Agitation
Due to ketamine’s dissociative properties, clinicians are increasingly using ketamine for treating pre-hospital and in-hospital agitation. Lacking a uniform definition for agitation, healthcare providers, institutions, and organizations may use different criteria to choose medication intervention in an agitated patient. Although the picture of agitation may change depending on the situation, validated scales like the Altered Mental Status Scale (AMSS) can define agitation’s severity.30 The AMSS translates agitation into a quantifiable, real concept. The line between agitation and delirium is often unclear but has major ramifications for a patient’s treatment and outcome.30 For example, excited delirium, an agitation subtype, classifies a patient’s agitation past the emotional component and includes psychomotor, metabolic, and contributing disease states as possible reasons for agitation.30
As with most psychiatric disorders, identifying and treating agitation has been suboptimal. Since the 1980s, a popular cocktail of medications, known among emergency department physicians as the “B-52” order, has been the mainstay of agitation treatment in psychiatric facilities and emergency departments.31 When examining the B-52 order’s components, it is easy to see the correlation between the regimen and the American jet-powered strategic bomber from which it derives its name: Benadryl 50 mg IM, haloperidol 5 mg IM, lorazepam 2 mg IM.31 The B-52 order serves as a reminder of the suboptimal approach traditionally taken when confronted with an agitated patient.
Ketamine’s different routes of administration have benefits and disadvantages. Although less invasive, oral ketamine takes a longer time to reach the therapeutic range, something that is undesirable in an overly aggressive patient. Intravenous administration has the quickest onset but is the most invasive. Securing IV access may not always be possible. The IM route is the most often used method for agitation control for its quick “on/off” onset and duration of action, and its applicable dosage form.
A review explains ketamine’s uses and benefits in comparison to other, more traditional agitation treatments.30 In terms of agitation efficacy, ketamine provides the same, if not better, response when compared to its more traditional counterparts.30 Ketamine has a significantly faster onset of action when compared to haloperidol (5 minutes versus 17 minutes) and requires less redosing (5% of patients re-dosed versus 20% of patients re-dosed, respectively).30 However, ketamine continues to show a higher incidence of adverse effects when compared to its anti-psychotic counterpart (percent incidence calculated from six studies where adverse events were recorded as a secondary outcome):30
- emergence reaction 12.3% (8/65 patients)
- An “emergence reaction” is an often hostile, psychiatric episode brought about by ketamine use
- hypersalivation 31.8% (22/69 patients)
- nausea and vomiting 8.5% (7/82 patients)
- respiratory complications 7.6% (9/118 patients)
Although studies report a higher incidence of adverse reactions when using ketamine for agitation, it is important to consider study limitations: small patient populations, co-administration of drugs, and lack of adverse event reporting (only half of 12 studies included adverse reactions).30 If used properly, ketamine can be a safe, quick-acting drug to stop agitation when compared to traditional treatments.
Some law enforcement agencies use ketamine. However, when they use ketamine improperly, or when adverse effects arise, ketamine can have dangerous consequences. Over a four-day period during late August of 2019 in Colorado, police gave 23-year-old Elijah McClain and 25-year-old Elijah McKnight excessive ketamine doses for agitation.32 McClain died from cardiac arrest and McKnight survived but required life support in the hospital.32 It is inappropriate to allow untrained police officers to inject ketamine as a law enforcement tool. However, police defend using ketamine saying suspects with mental health issues or suspects taking drugs can be belligerent and dangerous. A Minnesota whistleblower lawsuit filed by a former emergency medical services worker claims police pressured them to allow ketamine use uneccessarily.32 In Minneapolis, ketamine used by police rose from four incidents per year in 2015 to 62 in 2017.32 This marked increase in ketamine use is upsetting many healthcare professionals. Dr. Mary Dale Peterson, president of the American Society of Anesthesiologists, says that ketamine can have “dangerous complications,” just like any other anesthetic. Dr. Peterson points out that justifiably using ketamine occurs very rarely.32
Whistleblowers cite complications from unwarranted ketamine use are associated with emergence reactions, and improper dosages.32 McClain died when he was given a ketamine dose for a 200-pound man but only weighed 143 pounds.32 Pharmacists can play a role in educating other healthcare professionals about proper dosing and management of ketamine’s serious adverse effects.
Major Depressive Disorders
Generally, major depressive disorder’s (MDD) treatment focuses on pathophysiology and regulates serotonin, norepinephrine, and dopamine.32 Medications such as selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibits (SNRIs), tricyclics, tetracyclics, and serotonin modulators all target an increase in synaptic neurotransmitter levels.32 Unfortunately, these drugs are not consistently effective for all patients, require an 8-week trial period, and have unfavorable adverse effects. For many providers and their patients, MDD treatment can feel like an awful waiting game—one that they sometimes lose.
Ketamine is becoming increasingly popular for its use in treating refractory depression. However, it requires healthcare providers to understand how it works to avoid putting patients into a “K-hole.” It offers a different approach to the current FDA-approved drugs for MDD. Ketamine prevents glutamate reuptake; excess glutamate produces an antidepressant effect. Ketamine, at subanesthetic doses, produces euphoria, and improves symptoms within 24 hours after infusion.4,14,32,33 Depressive symptoms improve rapidly, but the effects last only a few days to weeks. As a result, ketamine is most useful as an adjunctive treatment option. Patients feel better for a brief period, giving their antidepressants a chance to start working.
In addition, prescribers have few options for patients with MDD who have suicidal behaviors and ideation. Ketamine seems promising for patients at an elevated risk for self-harm. The Ketamine for Rapid Reduction of Suicidal Thoughts in Major Depression trial examined ketamine’s potential benefits for 80 suicidal patients with MDD. The results of this randomized controlled study showed that ketamine was superior to midazolam in improving the Scale for Suicidal Ideation (SCI). Patients’ SCI scores improved 4.96 points within 24 hours after a ketamine infusion of 0.5 mg/kg over 40 minutes.32 This suggests clinical use of ketamine as an adjuvant agent for acute episodes of suicide ideation in patients maintained on guideline recommended therapy for MDD may be appropriate. However, patient safety remains a concern (e.g., dissociative effects, abuse potential, respiratory, and cardiovascular effects).
As mentioned earlier, esketamine (Spravato) is ketamine’s S-enantiomer and FDA-approved for treatment-resistant depression.33 In the TRANSFORM-1 randomized controlled trial, the antidepressant/esketamine groups did not have a statistically significant change in Montgomery-Asberg Depression Rating Scale (MADRS) total score (from baseline to study day 28) when compared to the antidepressant placebo group.34,35 However, the changes based on the MADRS were clinically meaningful and showed that esketamine has a beneficial role in treatment-resistant depression when used as an adjuvant agent.36,37 The combination of esketamine with an antidepressant produced desirable outcomes while minimizing adverse effects. Although adverse effects were low, several adverse effects are possible: vertigo, nausea, vomiting, anxiety, sedation, abuse potential, increased blood pressure, dissociation, and suicidal thoughts/behaviors.33
CONCLUSION
To paraphrase the father of toxicology, Paracelsus, it’s all about the dose. Ketamine is the poster child drug for this statement. Ketamine has the potential to be an important adjuvant therapy for the treatment of a range of conditions. Those listed in this CE—anesthesia, analgesia, and major depressive disorder—are currently the most studied disorders where ketamine and its derivatives may be useful. Due to ketamine’s dissociative and analgesic effects through NMDA antagonism, there may be additional future potential uses for ketamine in pain control and psychiatric disorders. Simply, ketamine treats not only the physical manifestations of these conditions but the emotional component that providers can easily overlook. However, the current data sets are small, many use rating scales instead of final health outcomes, and a larger and longer term series of trials are required to fully determine the place of ketamine in the treatment armamentarium for patients.
Pharmacists and other healthcare providers will need to distinguish between therapeutic use and addiction. Often, these lines are muddled. Providing education is a first step to preventing abuse. Usually, addiction is a manifestation of an untreated, or undertreated, medical condition. Pharmacist intervention helps patients and healthcare providers to make the safest, most informed decisions possible to ensure the best possible outcomes.
Pharmacist Post Test (for viewing only)
Pharmacist Post-Test
Objectives:
1. Identify patient populations in which ketamine use is justified based on its FDA approved indications and for off-labeled use where it has been sufficiently studied
2. Compare the different formulations of ketamine and its “kissing cousins”
3. Describe potential risks associated with ketamine use
1. Patient AV has a GCS score of 8 and requires intubation. He presents with volume depletion, hypotension, and sepsis. What drug would the anesthesiologist probably use for sedation?
a. Fentanyl
b. Etomidate
c. Ketamine
2. In which patients would you avoid recommending ketamine?
a. Patients with reactive airway disease
b. Patients with sepsis or hypotension
c. Patients with traumatic brain injury
3. Which formulation of esketamine is FDA-approved for treatment resistant depressive disorder?
a. Injectable
b. Nasal spray
c. Infusion
4. What is the most commonly used route of administration when using ketamine for agitation?
a. IV
b. IM
c. PO
5. A clinician asks you about ketamine’s adverse effects. What would you say to start?
a. Ketamine can cause cardiac arrythmias.
b. Ketamine can decrease blood pressure.
c. Ketamine can worsen peptic ulcers.
6. What is the correct order of administration for RSII medications?
a. Premedication, sedative, paralytic
b. Premedication, paralytic, sedative
c. Sedative, premedication, paralytic
7. When should prescribers monitor liver function in patients who receive repeated ketamine doses?
a. At baseline and every 1 to 2 months
b. At baseline and every 1 to 2 weeks
c. At baseline and every 1 to 2 days
8. What is a “K-hole?”
a. A networking experience
b. A near-death experience
c. An extraterrestrial experience
9. What is a limitation of using ketamine in MDD?
a. Depressive symptoms improve slowly
b. Requires an 8-week trial period first
c. Effects last only a few days to weeks
10. A police officer asks you to discuss ketamine and asks why you refer to similar drugs as “kissing cousins.” How would you explain it?
a. They all have similar potency and antagonize NMDA receptor
b. They are used in similar doses and act as a NMDA receptor agonist
c. They are structurally similar and antagonize NMDA receptor
Pharmacy Technician Post Test (for viewing only)
Technician Post-Test
Objectives:
1. Identify patient populations in which ketamine use is justified based on its FDA approved indications and for off-labeled use where it has been sufficiently studied
2. Compare the different formulations of ketamine and its “kissing cousins”
3. Describe potential risks associated with ketamine use
1. In which patient should prescribers avoid using ketamine?
a. patient with serious peptic ulcer
b. patient older than 3 months old
c. patient with uncontrolled hypertension
2. What ketamine dose results in dissociation?
a. 0.1 to 0.5 mg/kg
b. 0.5 to 2 mg/kg
c. 2 to 3.5 mg/kg
3. What is a risk associated with using ketamine in RSII?
a. adrenal suppression
b. increase ARDS incidence
c. emergent reactions
4. What ketamine formulation is currently available by prescription?
a. sublingual tablet
b. injectable solution
c. 24-hour patch
5. What risk is associated with ketamine use?
a. exacerbates underlying schizophrenia
b. lowers focal seizure threshold
c. increases incidence of ARDS
6. What is a key difference between PCP and ketamine?
a. PCP has a shorter duration of action than ketamine
b. PCP is 10 time more potent than ketamine
c. PCP has less severe psychiatric effects than ketamine
7. What is a benefit of using ketamine for agitation in comparison to haloperidol?
a. faster onset
b. more redosing
c. fewer side effects
8. What is ketamine’s FDA-approved indication?
a. agitation
b. analgesia
c. anesthesia
9. What can be expected when people use oral ketamine?
a. high bioavailability
b. high rate of adverse effects
c. low plasma peak concentrations
10. What is ketamine’s role in RSII?
a. premedication
b. sedative
c. paralytic
References
Full List of References
References
1. Li L, Vlisides PE. Ketamine: 50 Years of modulating the mind. Front Hum Neurosci. 2016;10:612. Published 2016 Nov 29. doi:10.3389/fnhum.2016.00612
2. Ketalar. Prescribing information. Par Pharmaceutical; 2022. Accessed July 25, 2022. https://www.parpharm.com/pdfs/catalog/sterile/Ketalar_PI_20220613.pdf
3. Institute of Medicine (US) Forum on Neuroscience and Nervous System Disorders. Glutamate-Related Biomarkers in Drug Development for Disorders of the Nervous System: Workshop Summary. Washington (DC): National Academies Press (US); 2011.
4. Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. Journal of Psychiatry Neuroscience. 2017;42(4):222-229. DOI: 10.1503/jpn.160175.
5. Vyklicky V, Korinek M, Smejkalova T, et al. Structure, function, and pharmacology of NMDA receptor channels. Physiol Res. 2014;63(Suppl 1):S191-S203. doi:10.33549/physiolres.932678
6. Godwin SA, Burton JH, Gerardo CJ, et al. American College of Emergency Physicians. Clinical policy: procedural sedation and analgesia in the emergency department. Ann Emerg Med. 2014;63(2):247-258.e18. doi:10.1016/j.annemergmed.2013.10.015[PubMed 24438649]
7. Ellingson A, Haram K, Sagen N, Solheim E. Transplacental passage of ketamine after intravenous administration. Acta Anaesthesiol Scand. 1977;21(1):41-44.[PubMed 842268]
8. Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006;60(7):341-348. doi:10.1016/j.biopha.2006.06.021
9. Zhu X, Kohan LR, Goldstein RB. substantial elevation of liver enzymes during ketamine infusion: a case report. A Pract. 2020;14(8):e01239. doi:10.1213/XAA.0000000000001239
10. Wilkinson ST, Sanacora G. Considerations on the Off-label Use of Ketamine as a Treatment for Mood Disorders. JAMA. 2017;318(9):793-794. doi:10.1001/jama.2017.10697
11. Ho JH, Dargan PI. Arylcyclohexamines (Ketamine, Phencyclidine, and Analogues). In: Critical Care Toxicology. Brent J, Burkhart K, Dargan P, Hatten B, Megarbane B, Palmer R, eds. Springer; 2016. https://doi.org/10.1007/978-3-319-20790-2_124-1
12. Lodge D, Mercier MS. Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacology. 2015;172(17):4254-4276. doi:10.1111/bph.13222
13. Study of Wafermine™ for post-bunionectomy or abdominoplasty pain. ClinicalTrials.gov identifier: NCT03246971. Updated July 23, 2018. Accessed Jul 25, 2022. https://clinicaltrials.gov/ct2/show/study/NCT03246971
14. Treating major depressive disorder: a quick reference guide. American Psychiatric Association. Published October 2010. Accessed July 25, 2022. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd-guide.pdf
15. Visser E, Schug SA. The role of ketamine in pain management. Biomed Pharmacother. 2006;60(7):341-348. doi:10.1016/j.biopha.2006.06.021
16. Lankenau SE, Clatts MC. Patterns of polydrug use among ketamine injectors in New York City. Subst Use Misuse. 2005;40(9-10):1381-1397. doi:10.1081/JA-200066936
17. Drug Fact Sheet: Ketamine. Department of Justice and Drug Enforcement Administration. Published April 2020. Accessed July 25, 2022. https://www.dea.gov/sites/default/files/2020-06/Ketamine-2020.pdf
18. Lankenau SE, Sanders B, Bloom JJ, et al. First injection of ketamine among young injection drug users (IDUs) in three U.S. cities. Drug Alcohol Depend. 2007;87(2-3):183-193. doi:10.1016/j.drugalcdep.2006.08.015
19. Average Cost of Illicit Street Drugs. AddictionResource.net. Updated June 21, 2021. Accessed July 25, 2022. https://www.addictionresource.net/cost-of-drugs/illicit/
20. Świądro M, Stelmaszczyk P, Lenart I, Wietecha-Posłuszny R. The Double Face of Ketamine-The Possibility of Its Identification in Blood and Beverages. Molecules. 2021;26(4):813. Published 2021 Feb 4. doi:10.3390/molecules26040813
21. Morris, H. Hamilton’s Pharmacopeia Ketamine: Realms and Realities. [Video]. Vice TV. December 26, 2017. Accessed July 25, 2022. https://www.vicetv.com/en_us/video/hamiltons-pharmacopeia-ketamine-realms-and-realities/59cd5d0b7752d1ac3e90aacf
22. Kurdi MS, Theerth KA, Deva RS. Ketamine: Current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283-290. doi:10.4103/0259-1162.143110
23. Scarponcini TR, Edwards CJ, Rudis MI, Jasiak KD, Hays DP. The role of the emergency pharmacist in trauma resuscitation. J Pharm Pract. 2011;24(2):146-159. doi:10.1177/0897190011400550
24. Lee M, Silverman SM, Hansen H, Patel VB, Manchikanti L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician. 2011;14(2):145-161.
25. Johnstone-Petty, M. Ketamine use for complex pain in the palliative care population. J Hosp Palliat Nurs. 2018;20(6):561-567. doi: 10.1097/NJH.0000000000000488.
26. Mercadante S, Caruselli A., Casuccio A. The use of ketamine in a palliative-supportive care unit: a retrospective analysis. Ann Palliat Med. 2018;7(2): 205-210. doi: 10.21037/apm.2018.01.01
27. Bell RF, Kalso EA. Ketamine for pain management. Pain Rep. 2018;3(5):e674. Published 2018 Aug 9. doi:10.1097/PR9.0000000000000674
28. Lalanne L, Nicot C, Lang JP, et al. Experience of the use of Ketamine to manage opioid withdrawal in an addicted woman: a case report. BMC Psychiatry. 2016;16(1):395. doi:10.1186/s12888-016-1112-2
29. Blonk MI, Koder BG, Van Den Bemt PMLA, Huygen FJPM. Use of oral ketamine in chronic pain management: a review. Eur J Pain. 2012;14(5): 466-472. https://doi-org.ezproxy.lib.uconn.edu/10.1016/j.ejpain.2009.09.005
30. Linder LM, Ross CA, Weant KA. Ketamine for the acute management of excited delirium and agitation in the prehospital setting. Pharmacotherapy. 2018;38(1):139-151. doi:10.1002/phar.2060
31. Lulla AA, Singh M. The Art of the ED Takedown. emDOCs.net - Emergency Medicine Education. Published March 4, 2015. Accessed July 25, 2022. http://www.emdocs.net/the-art-of-the-ed-takedown/
32. Young R, McMahon S. Some States Allow Authorities to Use Ketamine to Subdue Suspects in The Field. But Is It Safe? Some States Allow Authorities to Use Ketamine to Subdue Suspects in The Field. But Is It Safe? | Here & Now. Published September 8, 2020. Accessed July 25, 2022. https://www.wbur.org/hereandnow/2020/09/08/ketamine-police-safety-elijah-mcclain
33. Ketamine. In: Lexi-Drugs. Lexi-Comp, Inc. Updated July 20, 2022. Accessed July 25, 2022. http://usj-ezproxy.usj.edu:2099/lco/action/doc/retrieve/docid/patch_f/7135?cesid=a8n33eDrj1M&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3Dketamine%26t%3Dname%26acs%3Dfalse%26acq%3Dketamine#rfs
34. Montgomery-Asperg Depression Rating Scale. Accessed July 27, 2022. https://www.mdcalc.com/calc/4058/montgomery-asberg-depression-rating-scale-madrs
35. Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22(10):616-630. doi:10.1093/ijnp/pyz039
36. Spravato. Prescribing information. Janssen Pharmaceutical Companies; 2019. Accessed July 25, 2022. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SPRAVATO-pi.pdf
37. Fedgchin M, Trivedi M, Daly EJ, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22(10):616-630. doi:10.1093/ijnp/pyz039