Learning Objectives
After completing this application-based continuing education activity, pharmacists and technicians will be able to
- Describe the etiology and pathophysiology of dry eye disease (DED) and its impact on quality of life
- Identify available and emerging over-the-counter and prescription therapies to treat DED
- Optimize artificial tear selection based on patient-specific characteristics
- Infer when to refer patients to the pharmacist or an eye care provider for DED

Release Date: August 15, 2023
Expiration Date: August 15, 2025
Course Fee
Pharmacists: FREE
Pharmacy Technicians: FREE
This CE was funded by: Alcon Vision, LLC
ACPE UANs
Pharmacist: 0009-0000-23-030-H01-P
Pharmacy Technician: 0009-0000-23-030-H01-T
Session Codes
Pharmacist: 23YC30-TVX83
Pharmacy Technician: 23YC30-XVT99
Accreditation Hours
2.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-23-030-H01-P/T will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Jennifer Salvon, RPh
Clinical Pharmacist
Mercy Medical Center
Springfield, MA
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Ms. Salvon does not have any relationships with ineligible companies.
ABSTRACT
Dry eye disease (DED) is a multifactorial condition affecting the ocular surface and tear function. Symptoms include burning, itching, and watery eyes. DED affects millions of people in the United States. Many underlying factors contribute to DED making therapeutic management difficult. Left untreated, DED can result in visual changes affecting everyday activities such as reading and driving. Simple environmental changes often help alleviate symptoms. Before seeking healthcare professional assistance, many people self-treat with over-the-counter artificial tear products, leading to high costs and frustration. Treatment involves patient education, environmental and lifestyle modifications, topically applied products, and, in severe cases, surgical procedures. Several recently approved products offer alternative treatment approaches. A knowledgeable, informed pharmacy team is prepared to counsel patients on product choice and to make appropriate referrals contributing to better patient outcomes.
CONTENT
Content
INTRODUCTION
The feeling of grit under the eyelids is uncomfortable, annoying, and frustrating and can pose a serious health issue. This feeling, often accompanied by burning, itching, redness, and visual disturbances, is a symptom of keratoconjunctivitis sicca, otherwise known as dry eye disease (DED). At its simplest, DED is inflammation of the cornea and conjunctiva from tear hyperosmolarity (higher concentration of solutes like salts, sugars, or other dissolved particles) and tissue dryness. Left untreated, DED may result in severe eye inflammation, corneal ulcers, and vision loss.1
DED affects approximately 16.4 million people, or 6.8% of the United States (U.S.) adult population.2,3 DED is likely underreported and underdiagnosed, with estimates as high as 22.9 million adults experiencing symptoms.2 Researchers estimate DED’s global prevalence is as high as 50%.4
Despite this prevalence, experts began to recognize DED as a disease state only about 30 years ago.5,6 Initially described as a component of Sjogren’s syndrome (an autoimmune disease involving tear and saliva glands), DED emerged as a separate condition as ocular surface study progressed. The National Eye Institute first defined DED in 1995.1
The Tear Film and Ocular Surface Society (TFOS) is a non-profit organization focused on eye health research and education.5 In 2015, the Dry Eye Workshop II (DEWS II), organized by TFOS, examined multiple aspects of DED. The workshop updated the definition, diagnosis, and classification of DED, the disease’s impact, therapeutic management options, and clinical trial design.5
TFOS DEWS II defines DED as "… a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles."5 In simpler terms, DED occurs when the tear film, which keeps the eyes moist, becomes imbalanced, leading to problems like tear film instability, high concentration of substances in the tears, inflammation and damage on eye surface, and abnormal nerve sensations.
Many risk factors contribute to DED development (Table 1). Women are two to three times more likely to develop DED than men.3,4 Risk of developing DED increases with age. Adults aged 50 or older are three times more likely to develop symptoms than those 18 to 49 years old.2,3 However, DED’s incidence is rising steadily in the younger population, possibly due to increased disease awareness.3 Digital device use may also contribute. Studies show that using digital devices decreases blink rate and increases incomplete blinks, leading to ocular surface dryness and, ultimately, DED.4.7
Table 1. Dry Eye Disease Risk Factors1,4,5
| Modifiable | Non-modifiable |
| Androgen deficiency
Computer use Contact lens wear Environment Medications |
Age ≥ 50 years
Asian race Connective tissue diseases (e.g., rheumatoid arthritis, Sjogren’s syndrome, systemic lupus erythematosus) Diabetes Female sex Meibomian gland dysfunction |
DED impacts the American economy significantly. Several factors contribute to DED burden: direct costs of medical care, the impact of lost productivity, and the associated quality of life burden. In the U.S., estimates of direct medical costs exceed $3.84 billion, fueled by healthcare professional visits, pharmacologic therapies, and surgical procedures.4,8 The cost of lost productivity (i.e., time spent seeking and receiving treatment, avoidance of aggravating work environments, and inability to perform work due to visual changes) is even more substantial. One study estimates that these indirect costs total $11,302 per patient annually.8 If more than 16 million people have DED, that totals more than $150 billion annually.4,8
Beyond monetary costs associated with DED, the disease also affects vision-related quality of life (VR-QoL). As DED progresses, visual quality decreases. Individuals with DED are three times more likely to report visual difficulties than those without.4 This impacts many daily activities such as reading, driving, watching television, and smartphone use.4 DED-associated pain and discomfort, along with difficulty in activities of daily living, impact mental health negatively.8 A 2021 study examined self-reported health status and psychological burden in patients with DED. The study associated DED with having a negative self-perception of health status and experiencing increased psychological stress.9
A Deeper Look at DED
A better understanding of DED requires review of the surface anatomy of a healthy eye (see Figure 1). The eye's surface consists of the ocular surface and ocular adnexa (accessory anatomical parts).10 The ocular surface includes the cornea, conjunctiva (including goblet cells), and tear film. The ocular adnexa includes the eyelids, lacrimal and meibomian glands, tear ducts, and the connecting muscles and nerves.10
Figure 1. Eye Surface Anatomy and Tear Film Formation
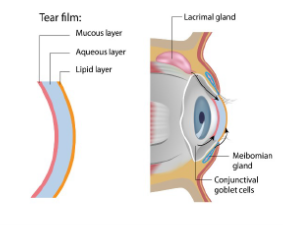
Tears lubricate the eye, and the tear film—which provides nutrients and moisture, removes microbes, and smooths the ocular surface—has three layers10,11:
- Outermost lipid layer, produced by meibomian glands
- Aqueous layer, produced by the lacrimal gland
- Innermost mucin layer, produced by goblet cells
Tear film instability, primarily increased tear osmolarity, leads to ocular surface damage in DED.7 DED's categorization is based on the mechanism leading to tear hyperosmolarity. In aqueous deficient dry eye disease (ADDE), decreased tear secretion increases tear film osmolarity. Increased evaporation of tears leads to hyperosmolarity in (you guessed it) evaporative dry eye disease (EDE).5
ADDE is further categorized based on the underlying cause: Sjogren’s Syndrome or non-Sjogren’s syndrome. As mentioned, Sjogren’s syndrome is an autoimmune disease attacking the salivary and lacrimal glands resulting in dry mouth and eyes. Non-Sjogren’s syndrome ADDE has various causes, including lacrimal deficiency, lacrimal gland duct obstruction, and systemic drugs. These mechanisms decrease tear secretion, resulting in tear hyperosmolarity.5,10
Meibomian gland dysfunction (MGD) is the primary cause of EDE.12,13 Meibomian glands line the inside of the upper and lower eyelid. Lipid secretion by meibomian glands forms a coating on the aqueous layer, impeding tear evaporation and providing protection against environmental irritants. Risk factors for MGD include aging, hormonal changes, contact lens wear, diet, and systemic and topical medications.13
Separation of DED into ADDE and EDE implies mutual exclusivity, but many patients presenting with DED exhibit characteristics of both. Recent evidence indicates the two classifications co-exist, with more patients presenting with EDE due to MGD. 6,14,15 Regardless of the subtype or mechanism, the result is a vicious, self-perpetuating cycle of inflammation.6,16 Tear film hyperosmolarity triggers an innate inflammatory immune response, activating CD4+ T-cells. This leads to conjunctival and corneal cell death and impaired lacrimal gland function, further decreasing tear production.16,17 This further increases tear hyperosmolarity, which continues the cycle.
Diagnosing DED is problematic due to its multi-factorial nature and inconsistent symptom presentation. Exploring differential diagnoses using triaging questions is crucial to exclude diseases that mimic DED, including allergic, bacterial, or viral conjunctivitis; blepharitis; and rheumatic disorders.5,18 A thorough patient history screens for risk factors such as smoking, contact lens wear, and certain systemic and topical medications. Several questionnaires also exist to help clinicians screen for DED. The Dry Eye Disease Questionnaire (DEQ-5) contains five items asking patients to rate the frequency of eye discomfort, eye dryness, and watery eyes during a typical day.18 The Ocular Surface Disease Index (OSDI) is another popular questionnaire. The OSDI questionnaire asks a series of 12 questions assessing eye symptoms, vision issues (e.g., reading, driving), and environmental conditions.18
Patients with positive questionnaire results should progress to a more detailed tear film and ocular surface examination. A positive result in any of the following tests is diagnostic of DED18:
- Tear breakup time (TBUT): There are two methods for measuring TBUT, using fluorescein dye or illumination of the cornea. Both measure how long it takes for tears to break up after a blink. Lower TBUT scores indicate tear instability.
- Osmolarity: Clinicians use a device with a test strip to gain a sample of the tear film from both eyes to check tear osmolarity. An osmolarity of 308 mOsm/L or greater in either eye or a difference of more than 8 mOsm/L between the eyes is diagnostic of DED.
- Ocular surface staining: After applying dye to the lower eyelid’s inner lining, clinicians examine the ocular surface for missing or damaged epithelial cells. Positive scores range from five to nine spots depending on the dye used.
Clinicians also commonly deploy the Schirmer test to evaluate the eye’s ability to produce tears. A notched paper strip placed over the lower eyelid stimulates tear production during the test. After five minutes, a length of wetting greater than 10 mm indicates normal tear function. Values less than 5 mm signify tear insufficiency.18
Pause and ponder: How would vision loss affect your everyday life?
Treatment Goals
Treatment goals for DED are to decrease ocular inflammation and restore ocular surface homeostasis (balance). DED's complexity and heterogeneous presentation necessitate an individualized approach. TFOS DEWS II recommendations emphasize identifying the disease’s root cause to determine an appropriate management approach.14 From there, the report outlines a stepwise, flexible approach to guide treatment based on patient-specific disease etiology and severity.14 Table 2 briefly summarizes recommended management steps.
Table 2. Treatment Steps in DED Management14
| Step 1:
· Education · Environmental modifications · Lifestyle modifications · Dietary supplementation · Eyelid hygiene · Medication review · Artificial Tears |
Step 2:
· Preservative-free artificial tears · Prescription therapy · Tear Conservation · Overnight treatments · In-office treatments |
| Step 3:
· Tear stimulation · Biological tear substitutes · Therapeutic contact lenses |
Step 4:
· Prescription therapy · Surgical intervention |
NON-PHARMACOLOGIC TREATMENT
One of the first steps, patient education, is essential for successful disease management.14 Patient education starts with thoroughly explaining DED’s chronic nature, including the ongoing, often long-term nature of therapeutic management. Discussing the patient’s home and work environment during the session may identify contributing factors.14 The environment affects overall health and well-being. Air pollution, low humidity, high altitude, and wind contribute to DED development.14 Adding an air humidifier inside or using protective eyeglasses outside can help mitigate DED symptoms. Other strategies include minimizing exposure to digital screens, cigarette smoke, and air conditioning.19
Proper lid hygiene is important in managing many eye conditions, including DED.14 Patients can use a cotton swab to scrub the eyelid with a dilute solution of baby shampoo to keep the area free of crusty build-up and environmental contaminants. Warm eye compresses also promote good lid hygiene and help alleviate DED symptoms. Unfortunately, lid hygiene adherence is poor, with estimates of just over 50% adherence at six weeks.14 Reinforcing the importance of lid hygiene with patients is an important component of DED patient counseling.
Identifying medications that may contribute to DED is an important task for pharmacy staff. Many medication classes produce drying effects on the body, intentionally or as an adverse effect.14,19 Table 3 lists examples of medications that may worsen DED. Pharmacists and pharmacy technicians should review patient profiles to identify drying medications, including ophthalmic formulations, as medications for glaucoma (an eye condition causing progressive vision loss) may contribute to DED.14 Mitigating options to consider include changing the route of administration from oral to topical, substituting with a therapeutic alternative, and adjusting doses.14
Table 3. Examples of Medications that Worsen Dry Eye Disease14,19
| Drug Class | Examples |
| Antihistamines and decongestants
|
Chlorpheniramine
Diphenhydramine Fexofenadine Loratadine Pseudoephedrine |
| Antidepressants
· TCA · SSRI · SNRI
|
Amitriptyline
Citalopram Duloxetine Fluoxetine Sertraline Venlafaxine |
| Anti-Parkinson’s | Levodopa |
| Antipsychotics
|
Aripiprazole
Perphenazine Quetiapine |
| Beta-blockers
|
Atenolol
Carvedilol Metoprolol Propranolol |
| Diuretics
|
Furosemide
Hydrochlorothiazide |
| Proton pump inhibitors
|
Omeprazole
Pantoprazole |
| Hormone therapy | Estrogen |
DED = dry eye disease; TCA = tricyclic antidepressants; SSRI = serotonin-selective reuptake inhibitor; SNRI = serotonin-norepinephrine reuptake inhibitor
Diet and Nutrition
An emerging body of evidence suggests that certain diet changes and nutritional supplementation may play a role in DED treatment. Dehydration increases tear osmolarity, so maintaining adequate hydration is important to disease control.14 Lactoferrin is an anti-inflammatory glycoprotein found in natural tears. Studies have found decreased lactoferrin levels in patients with DED leading researchers to the explore lactoferrin topical application and oral supplementation as treatment for the condition. One study found improved dry eye symptoms and tear film stability in patients taking an oral lactoferrin supplement.20 Oral lactoferrin is available as a supplement in many retail locations.
Supplementation with omega-3 fatty acids also shows potential in DED. Omega-3 fatty acids block proinflammatory substances and are essential for ocular surface homeostasis.14 Some studies have found that omega-3 fatty acid supplementation improves TBUT and Schirmer scores.21,22 Conversely, The Dry Eye Assessment and Management (DREAM) trial reported no difference between groups receiving omega-3 fatty acids and placebo.23 While oral omega-3 fatty acids show benefit for some patients, further study is necessary. These conflicting results prompted studies for alternative administration routes. Topical application of omega-3 fatty acids shows promise. A systematic review of 10 studies (five in animals and five in humans) showed overall improvement in ocular surface staining and TBUT.24 Further study is necessary to evaluate long-term efficacy and optimize dosage and delivery formulations.
Artificial Tears
Patients often attempt self-treatment before seeking healthcare professional assistance. Tear replacement with artificial tear (AT) formulations is essential for patient comfort and a mainstay of initial and ongoing therapy. Global sales of AT reached $2.64 billion in 2019, and experts predict this to reach $4.30 billion by 2027.25 Many AT products line the pharmacy shelves, all touting their ability to lubricate the eye. Faced with the confusing array of products, patients often employ a trial-and-error approach for AT selection, leading to high costs and frustration. Knowing the differences between ATs enhances the pharmacy team’s ability to counsel patients effectively.
AT supplementation is generally safe and well tolerated and associated adverse effects are mild, including blurred vision and ocular discomfort.14 Most ATs are water-based with viscosity-enhancing agents added for lubrication. Osmolarity, viscosity, and pH vary between products. Table 4 describes the components of AT products and their functions.
Table 4. Components of Artificial Tear Products11,14,26
| Component | Purpose | Examples |
| Viscosity-enhancing agents (lubricants) | Aid lubrication
Increase tear film thickness Protect ocular surface Promote tear retention Improve goblet cell density |
Carbomer 940 (polyacrylic acid)
Carboxymethyl cellulose (CMC) Dextran Glycerin Hyaluronic acid (HA) Hydroxypropyl-guar (HP-guar) Hydroxypropyl methylcellulose (HPMC) Polyvinyl alcohol Polyvinylpyrrolidone Polyethylene glycol (PEG) |
| Lipids
|
Restore the lipid layer
Increase lipid layer thickness Prevent evaporation |
Mineral oil
Castor oil Flaxseed oil |
| Osmoprotectants
|
Balance osmotic pressure
|
Trehalose
Levocarnitine Erythritol Betaine |
| Preservatives
|
Prevent microbial growth in multi-dose formulations | Benzalkonium chloride (BAK)
Sodium chlorite Sodium perborate |
| Buffers
|
Control pH | Sodium borate
Sodium citrate Sodium phosphate |
| Electrolytes
|
Promote ocular surface homeostasis | Potassium
Calcium Magnesium Phosphate |
Viscosity-enhancing agents, or demulcents, are the most common ingredient in AT and typically listed as the active ingredient on product packaging. The higher the viscosity (i.e., the thicker the product), the longer the ocular surface retention time, but differences in viscosity can influence product choice. High viscosity can create visual disturbances and buildup on the eyelid leading to decreased adherence.26 These products are best for nighttime use, and patients should use lower-viscosity products during the day.26 Many products contain multiple viscosity-enhancing agents. Commonly paired agents include carboxymethyl cellulose (CMC) with hyaluronic acid (HA) and hydroxypropyl-guar (HP-guar) with HA.14,26 Studies suggest that combining viscosity-enhancing components improves symptom control.14
There is significant interest in developing novel formulations to increase the spreading and retention time of applied drops.14 Lipid-containing eye drops are gaining in popularity as understanding of DED’s pathophysiology progresses.14 Lipids restore and thicken the lipid layer of the tear film and prevent tear evaporation. Formulated as oil-in-water emulsions, lipid-containing products contain macro-, micro-, or nano-particles. Particle size is important. Macro particles are associated with cloudy, blurred vision. As particle size decreases, blurring decreases.14
Osmoprotectants balance osmotic pressure, as the name implies, to protect and prevent corneal and conjunctival cell death.26 Levocarnitine and erythritol protect cells from hyperosmolar stress and improve DED’s symptoms.26 Clinical trials have shown that trehalose is more effective at improving ocular surface staining than saline.14,26
Multi-dose products contain preservatives to prevent microbial growth, but these can also worsen symptoms in DED. Benzalkonium chloride (BAK), the most common preservative, may cause corneal damage and interfere with tear film stability.14 Newer “disappearing preservatives” (e.g., sodium chlorite, sodium perborate) have a lower impact on the ocular surface. Exposure to light or the ocular surface breaks down these compounds, minimizing toxicity.14,27 Even newer preservatives carry risk, making preservative-free drops the best choice, especially in patients with severe DED. Preservative-free AT products are available in disposable single-use units but are generally more expensive.14
The pH of ATs affects product activity, stability, patient comfort, and safety.14 Adding electrolytes to reproduce the electrolyte profile of the tear film aids osmotic balance. Studies show that hypotonic solutions (i.e., having a lower osmotic pressure) decrease DED signs.26
No large-scale, randomized clinical trials have evaluated all currently available AT formulations. Some clinical trials evaluate individual AT products, and a few head-to-head studies exist.16,28 Several published systematic reviews have concluded that ATs treat DED safely and effectively. One systematic review of more than 60 studies published in 2022 drew the following conclusions27:
- Combination formulations, including the following, may be more effective than single-ingredient products: CMC and HA, HA and trehalose, CMC and glycerin, and HA and coenzyme Q10.
- Formulations containing polyethylene glycol (PEG) may be more effective than those with CMC.
- Preservative-free formulations are preferable.
- Patients with EDE and/or MGD should use drops containing phospholipids.
- Patients should administer AT four times daily for one month to assess efficacy.
- Patience is key; sometimes, it may take up to four months of consistent use to see improvement.
Another literature review of 18 studies compared commercially available AT products and concluded that products containing CMC, hydroxypropyl methylcellulose (HPMC), or HA were the most beneficial in improving patient comfort level.29 This study also determined that clinicians should recommend administration three to four times daily for two months to assess patient response before escalating therapy. The use of a preservative-free formulation is preferable.29 If patients choose or clinicians recommend preservative-containing eye drops, administration should be limited to four to six times daily.29 Researchers created a stepwise approach to selecting AT products29:
- Step 1: Start with CMC, HPMC, or HA-based formulations
- Step 2: Move to formulations with PEG or PEG and glycerin
- Step 3: Consider gel or lipid formulations
- Step 4: Progress to ointments, liposomal sprays, or prescription inserts
Both studies reached similar conclusions. Adherence and persistence are key to successfully evaluating an individual product, a fact that pharmacy staff should reiterate to patients. While some trial and error may be necessary, following the above recommendations allows patients and providers an organized approach to AT selection. While AT are a mainstay of early symptomatic treatment of dry eye disease, they do not address DED’s underlying causes. Prescription therapies target the underlying inflammatory processes.
Hydroxypropyl cellulose ophthalmic insert (HCOI) is a prescription-only lubricant insert containing 5 mg of hydroxypropyl cellulose. The insert is preservative-free and designed to provide continuous lubrication throughout the day. Patients insert HCOI once daily using an applicator.30 They rinse the applicator in hot water then use the grooved end to pick up the insert. Patients then place the insert in a pocket created by pulling out the outer corner of the eyelid. The HCOI softens and slowly dissolves, stabilizing and thickening the tear film, prolonging TBUT. One study comparing HCOI to using AT four or more times a day found increased TBUT and decreased foreign body sensation with HCOI compared to AT.30,31 Reported adverse effects include blurred vision, eye irritation, eyelid matting, and light sensitivity.30
Pause and Ponder: A patient approaches the pharmacy counter with a plastic bag full of bottles of different brands of artificial tears. Dumping them on the counter, she states, “None of these work! I don’t know what to do next.” What advice would you give her?
PRESCRIPTION THERAPIES
Available prescription therapies (outlined in Table 5) target the inflammatory cycle of DED through different mechanisms with varying degrees of success.
Table 5. Prescription Therapies to Treat Dry Eye Disease28,32-36
| Drug | Brand Name (Manufacturer) | Formulation(s) | Dosing | Supplied |
| Cyclosporine A | Restasis
(Allergan) |
0.05% emulsion | 1 drop in each eye BID | Single-use vials |
| Cequa
(Sun Pharma) |
0.09% solution | 1 drop in each eye BID | Single-use vials | |
| Generic
(Mylan) |
0.05% solution | 1 drop in each eye BID | Single-use vials | |
| Lifitegrast | Xiidra
(Novartis) |
5% solution | 1 drop in each eye BID | Single-use containers |
| Loteprednol | Eysuvis
(Kala Pharma) |
0.25% suspension | 1-2 drops in each eye QID for up to 2 weeks | Multi-dose 10 mL bottle |
| Perfluorohexyloctane | Meibo
(Bausch & Lomb) |
100% solution | 1 drop in each eye QID | Multi-dose 5 mL bottle |
| Varenicline | Tyrvaya
(Oyster Point Pharma) |
0.03 mg/0.05ml solution | 1 spray in each nostril BID | Multi-dose nasal spray |
ABBREVIATIONS: BID, twice daily; QID, four times daily
Cyclosporine A
Cyclosporine A (CsA) is an anti-inflammatory immune modulator approved for use in DED more than two decades ago.16 Calcineurin activates T-cells, increasing inflammatory cytokine production. CsA inhibits calcineurin to prevent T-cell activation, disrupting the inflammatory cycle in DED.16
Many clinical trials have evaluated CsA’s safety and efficacy in DED treatment.1,5,14,37 Results consistently show that CsA improves Schirmer test scores, corneal staining results, and goblet cell density. Improvement often takes several months, making patient education key to adherence.1,5,14,37 Topical CsA alleviates symptoms in approximately 50% of patients.1 Patients using CsA experience decreases in blurred vision, ocular dryness, foreign body sensation, and watery eyes.1,14 Treatment often causes stinging and irritation. Other adverse effects include blurred vision, ocular itching, eye redness, and foreign body sensation.37 Pretreatment with an ophthalmic steroid such as loteprednol may decrease CsA’s adverse effects.1,38
As a hydrophobic (water-fearing) substance, CsA is challenging to formulate into an ophthalmic topical formulation. Initially, products used castor oil and corn oil as vehicles, but poor bioavailability and adverse effects preclude their use.16 The first commercially available CsA product, a 0.05% emulsion, uses a castor oil-in-water emulsion, which reduces but does not eliminate adverse reactions.37
Approval of CsA 0.09% nanomicellar solution introduced a novel formulation.16,37 Clinical efficacy trials found a response as early as day 28.16At the end of 12 weeks, 17% of study participants receiving CsA 0.09% experienced increased tear production with a Schirmer score greater than 10 mm. Reported adverse effects included mild instillation site pain, eye irritation, blepharitis, and headache.32 Preliminary studies suggest CsA 0.09% is more effective and better tolerated than CsA 0.05%.16
Lifitegrast
Approved in 2016, the novel drug lifitegrast is a lymphocyte function-associated antigen-1 (LFA-1) antagonist.33 LFA-1 binds to intracellular adhesion molecule-1 during inflammation, activating T-cell migration and resulting in ocular inflammation. Lifitegrast binds to LFA-1, preventing this interaction and decreasing T-cell-mediated inflammation.33 The U.S. Food and Drug Administration (FDA) approved this drug based on four randomized, double-masked, 12-week efficacy and safety trials. 33,39-41 All studies showed a reduction in patient-rated eye dryness scores at the end of 12 weeks. Patients in three of the four studies experienced reduced corneal staining scores.33
The one-year multicenter, randomized, placebo-controlled SONATA study evaluated lifitegrast safety.42 Reported adverse effects included burning, reduced visual acuity, dry eye, and taste changes. Researchers observed no serious adverse events and discontinuation rates were 12.3% and 9% for lifitegrast and placebo, respectively.42 A 2021 retrospective review of 600 patient charts examined real-world experience with lifitegrast in DED.43 Most patients continued treatment for six months and showed improvement in DED symptoms. Patients also experienced improved quality of life at three and 12 months of treatment.43
Perfluorohexyloctane
Perfluorohexyloctane, formerly known as NOV3, reduces tear evaporation from the ocular surface.44 The drug’s exact mechanism in DED is unclear. In May 2023, the FDA approved an ophthalmic formulation containing perfluorohexyloctane for treating DED in adults 18 and older based on data from two phase 3 clinical trials: GOBI and MOJAVE.44,45
These trials evaluated efficacy and safety in more than 1,200 patients with DED meeting similar inclusion and exclusion criteria based on tear film break-up time, ocular surface disease scores, and MGD evaluations. Both trials were multi-center, randomized, double-masked, and saline-controlled.44,45 GOBE and MOJAVE results also consistently showed statistically significant reductions in reported symptoms of DED. Reported adverse events occurred in less than 4% of study participants and included blurred vision, blepharitis, instillation site pain, and conjunctival redness.44,46 Patients must remove contact lenses before and for at least 30 minutes after administration of perfluorohexyloctane drops.34
Perfluorohexyloctane should be available in the second half of 2023.44
Short-Term Corticosteroids
Corticosteroids are potent inhibitors of inflammatory mediators.14 Many clinical trials have demonstrated their efficacy in breaking the inflammatory cycle of DED. Unfortunately, long-term therapy is associated with increased intraocular pressure, cataracts, and risk of infection.14
Loteprednol is a synthetic corticosteroid derived from prednisolone. Its rapid breakdown into inactive metabolites reduces risk of adverse reactions.47 A retrospective safety study concluded that loteprednol therapy carries a low risk of treatment-related elevated intraocular pressure compared to other steroids.48 Several loteprednol ophthalmic formulations are available, but only the 0.25% suspension is FDA approved for the short-term treatment of DED. This formulation uses mucus-penetrating particle (MPP) technology to allow nanoparticle penetration through the mucin layer.47,49
The FDA approved loteprednol 0.25% suspension based on the STRIDE series of trials.36 These trials randomized patients with DED to the drug or a vehicle control four times daily in both eyes for two weeks. All trials reported significant improvements in eye redness and discomfort at the end of two weeks.36
One role for topical steroids in DED is pre-treatment prior to topical CsA therapy. A 2014 study compared loteprednol versus AT during a two-week lead-in period to CsA.38 Patients self-administered either loteprednol or AT four times daily for two weeks, followed by CsA twice daily plus either loteprednol or AT twice daily for an additional six weeks. Both groups showed improved ocular staining and OSDI and Schirmer scores. Loteprednol provided more rapid relief of DED symptoms and resulted in a lower CsA discontinuation rate than AT.38
Patients with moderate-to-severe DED with adequate long-term control may still experience periodic symptom exacerbation. Short-term pulse steroid therapy (using steroids of a week or two, then tapering and resuming if necessary) can be useful for patients with symptom exacerbations.14
Varenicline Nasal Spray
Pharmacy staff may recognize varenicline as a treatment for smoking cessation, but a newer nasal spray formulation shows utility for treating DED. Tear film production results from stimulating afferent nerves in the cornea and conjunctiva and parasympathetic nerves in the lacrimal gland, meibomian glands, and goblet cells.50,51 This neural pathway is accessible through central nervous system or peripherally through the nasal cavity. While the drug’s mechanism in DED is not fully understood, experts theorize that varenicline, a cholinergic agonist, activates this pathway to stimulate tear production.50,51
The randomized, double-masked, vehicle-controlled, 28-day ONSET-1 and ONSET-2 trials evaluated varenicline nasal spray’s safety and efficacy.50,51 Participants self-administered one spray of varenicline solution or vehicle in each nostril twice daily. Both studies found a significant improvement in tear production measured by Schirmer scores. The most common patient-reported adverse effects included sneezing, cough, throat irritation, and nasal irritation.50,51 The 2021 MYSTIC study examined varenicline nasal spray's long-term safety and efficacy compared to placebo over a 12-week period.52 Patients reported no severe or serious adverse events during the study; sneezing was the most common adverse reaction, occurring in 82% of patients.52
Varenicline packaging includes two glass bottles, each containing a 15-day drug supply. Patients must initially prime the bottle by pumping seven sprays into the air away from the face. Re-priming by pumping one spray into the air is necessary after five days of nonuse.35
Steps for administration of varenicline nasal spray35:
- Blow nose if needed to clear nostrils
- Remove the cap and clip from the bottle
- Hold the bottle upright, placing one finger on each side of the applicator and thumb on the bottom of the bottle
- Tilt head back slightly
- Insert the applicator tip into one nostril, pointing it toward the ear on the same side of the nostril, leaving space between the tip and the wall of the nostril
- Place tongue on roof of mouth and breath gently while pumping one spray into the nostril
- Repeat in other nostril
- Wipe the applicator with a clean tissue and replace the cap and clip
Antibiotics
Clinicians sometimes use oral or topical antibiotics with anti-inflammatory effects off-label to treat DED due to MGD.14 Many patients experience MGD due to overgrowth of eyelid flora, so reduction of eyelid flora and inflammation improves patient-reported symptoms.53 Oral administration of doxycycline and minocycline in small doses (40 to 400 mg of doxycycline and 50 to 100 mg of minocycline) to treat MGD improves patient-reported symptoms.1,53 Unfortunately, gastrointestinal adverse effects limit the use of these medications. One study found that azithromycin 1% eyedrops improved eyelid inflammation and tear film lipid layer stability.54
EMERGING THERAPIES
New and novel therapies are also in the pipeline for DED treatment. Pharmacy staff should be aware of their potential place in therapy and prepared to incorporate them upon approval.
Reproxalap
Exploring another causative mechanism in DED, reproxalap is a reactive aldehyde species (RASP) inhibitor. RASP molecules are found at the top of the inflammatory cascade and are elevated in many inflammatory diseases. They bind to and disrupt the function of enzymes and ion channels, which activates pro-inflammatory mediators. RASP inhibition, therefore, decreases pro-inflammatory substances associated with DED.55,56
A randomized, double-masked, phase 2a trial evaluated the efficacy of three formulations of reproxalap: 0.1% and 0.5% solutions and a 0.5% lipid solution.55 Participants used the products four times daily for 28 days. The study found a significant improvement in four questionnaire scores, Schirmer test values, tear osmolarity, and tear staining scores. Within one week, patients reported symptom improvement. Researchers concluded that reproxalap could potentially alleviate DED symptoms.55
A separate randomized, double-masked, phase 2b trial compared reproxalap 0.01% and 0.25% to a control vehicle solution.56 Patients self-administered drops four times daily for a total of 12 weeks. The study found statistically significant improvements in ocular dryness and staining over 12 weeks.56
A 2021 tolerability study compared ocular adverse effects between two formulations of reproxalap 0.25% (one solution, one lipid-based) and lifitegrast 5% solution.57 Over seven days, study participants received one dose of each solution with a 3-day washout period between administrations. Researchers assessed adverse effects after 1 hour. Reproxalap formulations were similar to one another and superior to lifitegrast in ocular discomfort, blurry vision, and dysgeusia.57
Reproxalap offers a novel approach to treating the underlying inflammatory process involved in DED. Preliminary study results show improvements in DED symptoms and better patient tolerability, potentially leading to lower discontinuation rates and improved patient outcomes.
Cationic Cyclosporine
A cationic (positively charged) 0.1% CsA nanoemulsion is available in Europe to treat DED.58 Experts theorize that a cationic emulsion increases the surface time of CsA on ocular tissues. All FDA-approved products are anionic (negatively charged). Clinical trials are evaluating CsA 0.1% nanoemulsion for FDA approval.58
PHARMACY TEAMS: FRONT-LINE SUPPORT
Pharmacists and pharmacy technicians are among the most accessible healthcare providers. People routinely turn to neighborhood pharmacies for advice on many health topics. Most people self-treat dry eye symptoms long before seeking professional help. These facts make the pharmacy team essential in supporting people suffering from DED. The SIDEBAR provides basic counseling information about eye products.
SIDEBAR: Counseling Tips for Eyedrop and Eye Ointment Administration59,60
Proper administration of ophthalmic formulations is key to their success. Administration is awkward, and many patients struggle with it. Advising patients on proper technique is a key role for the pharmacy team. General tips for all ophthalmic products include
- Confirm you have the correct product
- Check expiration date
- Read the directions
- Wash your hands
- If using both eyedrops and eye ointment, wait five to ten minutes between drops, and administer the eyedrops at least 10 minutes before the ointment
- Using a mirror may make it easier to see what you are doing
Eyedrops:
- Gently shake the bottle
- Be sure the eye dropper is clean, and do not let it touch any surface
- Tilt your head back and look up
- Pressing your finger gently on the skin just beneath the lower eyelid, pull your lower eyelid down and away from your eyeball to make a “pocket” for the drops
- With the other hand, hold the eye drop bottle upside down with the tip just above the pocket
- Squeeze the prescribed number of eye drops into the pocket
- If you think you did not get the drop of medicine into your eye properly, use another drop
- Blink a few times so that the medicine spreads across your eye
- For at least 1 minute, close your eye and press your finger lightly on your tear duct (small hole in the inner corner of your eye) to keep the eye drop from draining into your nose
- Wash your hands
- Wait at least 10 minutes before you use other eye products, especially ointments, gels, or other thick eye drops
Eye ointment:
- Be sure the top of the ointment tube is clean, and don’t let it touch any surface, including the eye, eyelid, or lashes. (If it does, call your pharmacy and arrange to get another tube of eye ointment.)
- Tilt your head back and look up
- With one hand, pull the lower eyelid down with one or two fingers to create a small pouch
- With the other hand, position the medicine above your eye
- Put a thin line of ointment in the pouch. Close the eye for 30 to 60 seconds to let the ointment absorb
- Wash your hands
- Eye ointments can cause some temporary blurring of vision
Knowledge of risk factors, including precipitating medications (revisit Table 3 for a refresher), aids in identifying patients at risk for developing DED. Technicians are often the first point of contact at the pharmacy counter, routinely fielding questions. Actively listening and asking open-ended questions help gather necessary information. Patients reporting dry eye symptoms or buying AT products may need counseling or a referral to a pharmacist or an eye care professional.
Educating patients about avoiding certain environmental factors is important. Remind patients that minimizing exposure to wind or smoke, taking a break from digital screens, and using a humidifier may help alleviate symptoms. Adherence to therapeutic interventions is key in DED treatment. Some interventions, such as lid hygiene, are time-consuming, and many patients stop after only a few days. Reinforcing the importance of lid hygiene with patients is an important component of DED patient counseling.
Advising patients on selecting an appropriate AT product decreases frustration and increases overall patient satisfaction. Proper administration of ophthalmic preparations can be difficult for some patients, particularly older individuals. Taking the time to counsel on proper technique sets patients up for successful administration and improved outcomes.
Patients with severe refractory DED may not benefit enough from lifestyle modifications and pharmacologic therapy. Many other interventions exist including14
- Punctal plugs blocking the tear ducts to promote tear conservation
- Pulsed light therapy delivered in office with a handheld flash gun
- Tear stimulation utilizing topical and systemic secretagogues
- Biological tear substitutes utilizing patient-derived serum
- Use of therapeutic contact lenses made of silicone hydrogel
- Surgery to correct any causative physiological abnormalities
Pharmacy staff should recognize when patients with worsening DED symptoms may require escalation of therapy and refer them to an eye care provider when appropriate.
Pause and Ponder: Consider your home and work environment. Could you take steps to minimize conditions contributing to developing dry eye?
CONCLUSION
You may have noticed a recurring theme throughout this activity: education. Helping patients understand the chronic nature of DED and navigate treatment options improves patient care and outcomes. Education must include the entire pharmacy team. Understanding the roles of each treatment allows for effective management and counseling. Educated pharmacy teams can assist patients with product selection, counsel on the timing and administration of treatments, improve safety, and provide referrals when appropriate.
Pharmacist Post Test (for viewing only)
The Nitty Gritty: Dry Eye Guidance for the Pharmacy Team
Posttest
Learning Objectives:
1. Describe the etiology and pathophysiology of dry eye disease (DED) and its impact on quality of life
2. Identify available and emerging over-the-counter and prescription therapies to treat DED
3. Optimize artificial tear selection based on patient-specific characteristics
4. Infer when to refer patients to the pharmacist or an eye care provider for DED
Pharmacists:
1. Which of the following is a risk factor for developing DED?
A. Caucasian race
B. Digital device use
C. Obesity
2. How does meibomian gland disease (MGD) contribute to DED?
A. Decreased lipid secretion affecting the outer layer of the tear film
B. Increased lipid secretion affecting the outer layer of the tear film
C. Decreased tear secretion leading to tear film instability
3. Prince Charming shares with you his recent DED diagnosis. Which of the following medications in his profile is most likely contributing to his symptoms?
A. Duloxetine
B. Donepezil
C. Erythromycin
4. Which of the following is a function of viscosity-enhancing agents in artificial tears?
A. Balance osmotic pressure
B. Control pH
C. Increase lubrication
5. Olaf stops by the pharmacy asking for assistance selecting an artificial tear product. He describes mild dry eye symptoms he is experiencing with the change in seasons. As a first choice, you suggest a product containing which of the following?
A. Carboxymethylcellulose (CMC)
B. CMC and hyaluronic acid (HA)
C. Polyvinyl alcohol
6. Which of the following is the most appropriate way to advise Olaf to use the recommended AT product to effectively manage symptoms and assess efficacy?
A. Apply 1-2 drops in each eye 1-2 times a day for 4-6 months
B. Apply 1-2 drops in each eye 4-6 times a day for 1-2 weeks
C. Apply 1-2 drops in each eye 3-4 times a day for 1-2 months
7. Buzz Lightyear recently received a diagnosis of DED due to MGD. Which of the following would be an appropriate first-line treatment choice?
A. Artificial tears formulated with mineral oil
B. Loteprednol 0.25% ophthalmic suspension
C. Oral omega-3 fatty acid supplements
8. Elsa started using cyclosporine A 0.05% eye drops for DED last month. While picking up her first refill, she mentions the drops are controlling her symptoms well but causing a burning sensation when she administers them. Which of the following is the most appropriate response?
A. Let her know this is a known adverse effect and to continue therapy as prescribed
B. Recommend she stop using the drops immediately, as she may be harming her eyes
C. Offer to contact her eye care provider to switch to cyclosporine A 0.09%
9. Which of the following is a novel eye drop approved for long-term use in DED?
A. Perfluorohexyloctane
B. Varenicline
C. Loteprednol
10. Snow White frequently stops by the pharmacy to ask for guidance about treating her DED. Today she shared that her AT is no longer working, and it’s the fifth one she has tried. You confirm she is properly and consistently administering ATs. Which of the following is the BEST recommendation for Snow White?
A. Assist her in selecting a more appropriate AT product to try based on trial-and-error
B. Advise her to reach out to her ophthalmologist to explore prescription therapies
C. Tell her that she must move out of the dusty cabin she shares with the seven dwarves
Pharmacy Technician Post Test (for viewing only)
Pharmacy Technicians:
1. Dry eye disease (DED) affects approximately how many U.S. adults?
A. 7 million
B. 16 million
C. 23 million
2. Which of the following is a risk factor for developing DED?
A. Caucasian race
B. Digital device use
C. Obesity
3. Prince Charming shares his recent diagnosis of DED. Which of the following medications in his profile may be a contributing factor?
A. Duloxetine
B. Donepezil
C. Erythromycin
4. Why is it important to engage with patients at the counter and ask open-ended questions?
A. So you can stay updated with their vacation plans and get some destination ideas
B. To help gather important health-related patient information and optimize therapy
C. It’s not important; the patient wants to pick up their prescription as quickly as possible
5. Which of the following is a function of viscosity-enhancing agents in artificial tears?
A. Balance osmotic pressure
B. Control pH
C. Increase lubrication
6. Cinderella approaches the register with two open bottles of AT and a receipt from one week ago. She asks if she can return the products, as they did not work. Which of the following is the BEST response?
A. Refer Cinderella to the front end of the store to process the refund
B. Issue Cinderella a refund and suggest she speak to an ophthalmologist
C. Refer Cinderella to the pharmacist for counseling
7. Buzz Lightyear stops at the counter to purchase artificial tear eye drops. When he asks you how to use them, what should you do?
A. Tell him to follow the directions on the box; they clearly outline how to use them
B. Offer Buzz a patient handout explaining eye drop use, and refer him to the pharmacist
C. Explain that his doctor is the best person to educate him about eye drop administration
8. Olaf stops by the pharmacy, complaining that his eyes always feel dry, especially when he is outside sledding. Which of the following is the BEST suggestion?
A. Wear eye protection when sledding to reduce wind exposure
B. Watch YouTube videos of other people sledding instead
C. Build a snowman friend on top of the mountain and play there
9. Elsa seems quieter than usual when picking up her prescriptions. When you ask her if everything is OK, she shares that it feels like something is in her eye all the time and she is having a hard time reading her book for book club. Which of the following is the BEST response?
A. Suggest that she get the audiobook instead so she can still enjoy her book club
B. Let her know that this is common and over-the-counter therapies may help
C. Recommend that she see an eye care provider to prescribe loteprednol eye drops
10. While picking up a prescription, Snow White also purchases 4 bottles of artificial tears, stating that she goes through them like water. When you ask her if they help, she replies “Eh, not really…” How should you respond?
A. Tell her to keep it up; sometimes, artificial tears take a while to work
B. Explain that this isn’t typical and refer her to the pharmacist for counseling
C. Let her know it’s okay to stop using them if they aren’t working
References
Full List of References
References
References
1. Mohamed HB, Abd El-Hamid BN, Fathalla D, Fouad EA. Current trends in pharmaceutical treatment of dry eye disease: A review. Eur J Pharm Sci. 2022;175:106206. doi:10.1016/j.ejps.2022.106206
2. Farrand KF, Fridman M, Stillman IÖ, Schaumberg DA. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am J Ophthalmol. 2017;182:90-98. doi:10.1016/j.ajo.2017.06.033
3. Dana R, Bradley JL, Guerin A, et al. Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health Care System. Am J Ophthalmol. 2019;202:47-54. doi:10.1016/j.ajo.2019.01.026
4. Stapleton F, Alves M, Bunya VY, et al. TFOS DEWS II Epidemiology Report. Ocul Surf. 2017;15(3):334-365. doi:10.1016/j.jtos.2017.05.003
5. Craig JP, Nelson JD, Azar DT, et al. TFOS DEWS II Report Executive Summary. Ocul Surf. 2017;15(4):802-812. doi:10.1016/j.jtos.2017.08.003
6. Craig JP, Nichols KK, Akpek EK, et al. TFOS DEWS II Definition and Classification Report. Ocul Surf. 2017;15(3):276-283. doi:10.1016/j.jtos.2017.05.008
7. Al-Mohtaseb Z, Schachter S, Shen Lee B, Garlich J, Trattler W. The Relationship Between Dry Eye Disease and Digital Screen Use. Clin Ophthalmol. 2021;15:3811-3820. Published 2021 Sep 10. doi:10.2147/OPTH.S321591
8. McDonald M, Patel DA, Keith MS, Snedecor SJ. Economic and Humanistic Burden of Dry Eye Disease in Europe, North America, and Asia: A Systematic Literature Review. Ocul Surf. 2016;14(2):144-167. doi:10.1016/j.jtos.2015.11.002
9. Wang MT, Muntz A, Wolffsohn JS, Craig JP. Association between dry eye disease, self-perceived health status, and self-reported psychological stress burden. Clin Exp Optom. 2021 Nov;104(8):835-840. doi: 10.1080/08164622.2021.1887580. Epub 2021 Mar 3. PMID: 33689664.
10. Clayton JA. Dry Eye. N Engl J Med. 2018;378(23):2212-2223. doi:10.1056/NEJMra1407936
11. Kathuria A, Shamloo K, Jhanji V, Sharma A. Categorization of Marketed Artificial Tear Formulations Based on Their Ingredients: A Rational Approach for Their Use. J Clin Med. 2021;10(6):1289. Published 2021 Mar 21. doi:10.3390/jcm10061289
12. Bron AJ, de Paiva CS, Chauhan SK, et al. TFOS DEWS II pathophysiology report [published correction appears in Ocul Surf. 2019 Oct;17(4):842]. Ocul Surf. 2017;15(3):438-510. doi:10.1016/j.jtos.2017.05.011
13. Chhadva P, Goldhardt R, Galor A. Meibomian Gland Disease: The Role of Gland Dysfunction in Dry Eye Disease. Ophthalmology. 2017;124(11S):S20-S26. doi:10.1016/j.ophtha.2017.05.031
14. Jones L, Downie LE, Korb D, et al. TFOS DEWS II Management and Therapy Report. Ocul Surf. 2017;15(3):575-628. doi:10.1016/j.jtos.2017.05.006
15. Lemp MA, Crews LA, Bron AJ, Foulks GN, Sullivan BD. Distribution of aqueous-deficient and evaporative dry eye in a clinic-based patient cohort: a retrospective study. Cornea. 2012;31(5):472-478. doi:10.1097/ICO.0b013e318225415a
16. de Oliveira RC, Wilson SE. Practical guidance for the use of cyclosporine ophthalmic solutions in the management of dry eye disease. Clin Ophthalmol. 2019;13:1115-1122. Published 2019 Jul 1. doi:10.2147/OPTH.S184412
17. Pflugfelder SC, de Paiva CS. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology. 2017;124(11S):S4-S13. doi:10.1016/j.ophtha.2017.07.010
18. Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II Diagnostic Methodology report. Ocul Surf. 2017;15(3):539-574. doi:10.1016/j.jtos.2017.05.001
19. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-82.
20. Vagge A, Senni C, Bernabei F, et al. Therapeutic Effects of Lactoferrin in Ocular Diseases: From Dry Eye Disease to Infections. Int J Mol Sci. 2020;21(18):6668. Published 2020 Sep 12. doi:10.3390/ijms21186668
21. Bhargava R, Kumar P, Kumar M, Mehra N, Mishra A. A randomized controlled trial of omega-3 fatty acids in dry eye syndrome. Int J Ophthalmol. 2013;6(6):811-816. Published 2013 Dec 18. doi:10.3980/j.issn.2222-3959.2013.06.13
22. Liu A, Ji J. Omega-3 essential fatty acids therapy for dry eye syndrome: a meta-analysis of randomized controlled studies. Med Sci Monit. 2014;20:1583-1589. Published 2014 Sep 6. doi:10.12659/MSM.891364
23. Zhao M, Yu Y, Ying GS, Asbell PA, Bunya VY; Dry Eye Assessment and Management Study Research Group. Age Associations with Dry Eye Clinical Signs and Symptoms in the Dry Eye Assessment and Management (DREAM) Study. Ophthalmol Sci. 2023;3(2):100270. Published 2023 Jan 12. doi:10.1016/j.xops.2023.100270
24. Paik B, Tong L. Topical Omega-3 Fatty Acids Eyedrops in the Treatment of Dry Eye and Ocular Surface Disease: A Systematic Review. Int J Mol Sci. 2022;23(21):13156. Published 2022 Oct 29. doi:10.3390/ijms232113156Intro
25. Pharmaceutical. Artificial Tears Market Size, Share & COVID-19 Impact Analysis. Available online at: https://www.fortunebusinessinsights.com/artificial-tears-market-103486 (Accessed June 5, 2023)
26. Labetoulle M, Benitez-Del-Castillo JM, Barabino S, et al. Artificial Tears: Biological Role of Their Ingredients in the Management of Dry Eye Disease. Int J Mol Sci. 2022;23(5):2434. Published 2022 Feb 23. doi:10.3390/ijms23052434
27. Semp DA, Beeson D, Sheppard AL, Dutta D, Wolffsohn JS. Artificial Tears: A Systematic Review. Clin Optom (Auckl). 2023;15:9-27. Published 2023 Jan 10. doi:10.2147/OPTO.S350185
28. Restasis [package insert]. Irvine, CA: Allergan. Accessed June 5, 2023. https://media.allergan.com/actavis/actavis/media/allergan-pdf-documents/product-prescribing/Combined-Restasis-and-MultiDose-PI_8-3-17.pdf
29. Moshirfar M, Pierson K, Hanamaikai K, Santiago-Caban L, Muthappan V, Passi SF. Artificial tears potpourri: a literature review. Clin Ophthalmol. 2014;8:1419-1433. Published 2014 Jul 31. doi:10.2147/OPTH.S65263
30. Lacrisert (hydroxypropyl cellulose ophthalmic insert). Accessed June 23, 2023. https://www.lacrisert.com
31. Lacrisert [package insert]. Bridgewater, NJ: Bausch & Lomb; 2019. Accessed June 23, 2023. https://www.lacrisert.com/siteassets/pdf/Lacrisert-package-insert.pdf
32. Cequa [package insert]. Cranberry, NJ: Sun Pharmaceuticals. Accessed June 5, 2023. https://www.cequapro.com/CequaPI.pdf
33. Xiidra [package insert]. East Hannover, NJ: Novartis. 2020. Accessed June 1, 2023. https://www.novartis.com/us-en/sites/novartis_us/files/xiidra.pdf
34. Meibo [package insert]. Bridgewater, NJ: Bausch & Lomb. 2023. Accessed June 5, 2023. https://www.bausch.com/globalassets/pdf/packageinserts/pharma/miebo-package-insert.pdf
35. Tyrvaya [package insert]. Princeton, NJ: Oyster Point Pharma. 2021. Accessed June 5, 2023. https://www.tyrvaya-pro.com/files/prescribing-information.pdf
36. Eysuvis [package insert]. Watertown, MA: Kala Pharmaceuticals. Accessed June 14, 2023. https://www.eysuvis-ecp.com/pdf/prescribing-information.pdf
37. Periman LM, Perez VL, Saban DR, Lin MC, Neri P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J Ocul Pharmacol Ther. 2020;36(3):137-146. doi:10.1089/jop.2019.0060
38. Sheppard JD, Donnenfeld ED, Holland EJ, et al. Effect of loteprednol etabonate 0.5% on initiation of dry eye treatment with topical cyclosporine 0.05%. Eye Contact Lens. 2014;40(5):289-296. doi:10.1097/ICL.0000000000000049
39. Sheppard JD, Torkildsen GL, Lonsdale JD, et al. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: results of the OPUS-1 phase 3 study. Ophthalmology. 2014;121(2):475-483. doi:10.1016/j.ophtha.2013.09.015
40. Tauber J, Karpecki P, Latkany R, et al. Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study. Ophthalmology. 2015;122(12):2423-2431. doi:10.1016/j.ophtha.2015.08.001
41. Holland EJ, Luchs J, Karpecki PM, et al. Lifitegrast for the Treatment of Dry Eye Disease: Results of a Phase III, Randomized, Double-Masked, Placebo-Controlled Trial (OPUS-3). Ophthalmology. 2017;124(1):53-60. doi:10.1016/j.ophtha.2016.09.025
42. Donnenfeld ED, Karpecki PM, Majmudar PA, et al. Safety of Lifitegrast Ophthalmic Solution 5.0% in Patients With Dry Eye Disease: A 1-Year, Multicenter, Randomized, Placebo-Controlled Study. Cornea. 2016;35(6):741-748. doi:10.1097/ICO.0000000000000803
43. Hovanesian JA, Nichols KK, Jackson M, et al. Real-World Experience with Lifitegrast Ophthalmic Solution (Xiidra®) in the US and Canada: Retrospective Study of Patient Characteristics, Treatment Patterns, and Clinical Effectiveness in 600 Patients with Dry Eye Disease. Clin Ophthalmol. 2021;15:1041-1054. Published 2021 Mar 8.
44. Tauber J, Berdy GJ, Wirta DL, Krösser S, Vittitow JL; GOBI Study Group. NOV03 for Dry Eye Disease Associated with Meibomian Gland Dysfunction: Results of the Randomized Phase 3 GOBI Study. Ophthalmology. 2023;130(5):516-524. doi:10.1016/j.ophtha.2022.12.021
45. Bausch + Lomb News Releases. www.bausch.com. Accessed June 9, 2023. https://www.bausch.com/news/releases/?id=156
46. Sheppard JD, Kurata F, Epitropoulos AT, Krösser S, Vittitow JL; MOJAVE Study Group. NOV03 for Signs and Symptoms of Dry Eye Disease Associated With Meibomian Gland Dysfunction: The Randomized Phase 3 MOJAVE Study [published online ahead of print, 2023 Mar 21]. Am J Ophthalmol. 2023;252:265-274. doi:10.1016/j.ajo.2023.03.008
47. Venkateswaran N, Bian Y, Gupta PK. Practical Guidance for the Use of Loteprednol Etabonate Ophthalmic Suspension 0.25% in the Management of Dry Eye Disease. Clin Ophthalmol. 2022;16:349-355. Published 2022 Feb 9. doi:10.2147/OPTH.S323301
48. Sheppard JD, Comstock TL, Cavet ME. Impact of the Topical Ophthalmic Corticosteroid Loteprednol Etabonate on Intraocular Pressure. Adv Ther. 2016;33(4):532-552. doi:10.1007/s12325-016-0315-8
49. Gupta PK, Venkateswaran N. The role of KPI-121 0.25% in the treatment of dry eye disease: penetrating the mucus barrier to treat periodic flares. Ther Adv Ophthalmol. 2021;13:25158414211012797. Published 2021 May 5. doi:10.1177/25158414211012797
50. Wirta D, Torkildsen GL, Boehmer B, et al. ONSET-1 Phase 2b Randomized Trial to Evaluate the Safety and Efficacy of OC-01 (Varenicline Solution) Nasal Spray on Signs and Symptoms of Dry Eye Disease. Cornea. 2022;41(10):1207-1216. doi:10.1097/ICO.0000000000002941
51. Wirta D, Vollmer P, Paauw J, et al. Efficacy and Safety of OC-01 (Varenicline Solution) Nasal Spray on Signs and Symptoms of Dry Eye Disease: The ONSET-2 Phase 3 Randomized Trial. Ophthalmology. 2022;129(4):379-387. doi:10.1016/j.ophtha.2021.11.004
52. Quiroz-Mercado H, Hernandez-Quintela E, Chiu KH, Henry E, Nau JA. A phase II randomized trial to evaluate the long-term (12-week) efficacy and safety of OC-01 (varenicline solution) nasal spray for dry eye disease: The MYSTIC study. Ocul Surf. 2022;24:15-21. doi:10.1016/j.jtos.2021.12.007
53. Thulasi P, Djalilian AR. Update in Current Diagnostics and Therapeutics of Dry Eye Disease. Ophthalmology. 2017;124(11S):S27-S33. doi:10.1016/j.ophtha.2017.07.022
54: Arita R, Fukuoka S. Efficacy of Azithromycin Eyedrops for Individuals With Meibomian Gland Dysfunction-Associated Posterior Blepharitis. Eye Contact Lens. 2021;47(1):54-59. doi:10.1097/ICL.0000000000000729
55. Clark D, Sheppard J, Brady TC. A Randomized Double-Masked Phase 2a Trial to Evaluate Activity and Safety of Topical Ocular Reproxalap, a Novel RASP Inhibitor, in Dry Eye Disease. J Ocul Pharmacol Ther. 2021;37(4):193-199. doi:10.1089/jop.2020.0087
56. Clark D, Tauber J, Sheppard J, Brady TC. Early Onset and Broad Activity of Reproxalap in a Randomized, Double-Masked, Vehicle-Controlled Phase 2b Trial in Dry Eye Disease. Am J Ophthalmol. 2021;226:22-31. doi:10.1016/j.ajo.2021.01.011
57. McMullin D, Clark D, Cavanagh B, Karpecki P, Brady TC. A Post-Acute Ocular Tolerability Comparison of Topical Reproxalap 0.25% and Lifitegrast 5% in Patients with Dry Eye Disease. Clin Ophthalmol. 2021;15:3889-3900. Published 2021 Sep 22. doi:10.2147/OPTH.S327691
58. Gupta PK, Asbell P, Sheppard J. Current and Future Pharmacological Therapies for the Management of Dry Eye. Eye Contact Lens. 2020;46 Suppl 2:S64-S69. doi:10.1097/ICL.0000000000000666
59. How to Put in Eye Drops. National Eye Institute. Accessed May 30, 2023. https://www.nei.nih.gov/Glaucoma/glaucoma-medicines/how-put-eye-drops
60. Eye Problems: Using Eyedrops and Eye Ointment. Kaiser Permanente. Accessed May 30, 2023. https://healthy.kaiserpermanente.org/health-wellness/health-encyclopedia/he.eye-problems-using-eyedrops-and-eye-ointment.za1098