Learning Objectives
| 1. Differentiate UC from Crohn’s disease |
| 2. Describe currently available and novel UC medications under development in the United States |
| 3. Outline the relationship between the types of treatment for UC, patient characteristics and disease severity |
| 4. Identify patient education pearls to address inflammation and advance to remission |
| 1. Differentiate UC from Crohn’s disease |
| 2. Describe currently available and novel UC medications under development in the United States |
| 3. Outline the relationship between the types of treatment for UC, patient characteristics and disease severity |
| 4. List symptoms that a patient with UC may share with a pharmacy technician |
Release Date:
Release Date: May 15, 2023
Expiration Date: May 15, 2026
Course Fee
FREE
This CE was funded by an educational grant from Bristol Meyer Squibb
ACPE UANs
Pharmacist: 0009-0000-23-014-H01-P
Pharmacy Technician: 0009-0000-23-014-H01-T
Session Codes
Pharmacist: 23YC14-HTX49
Pharmacy Technician: 23YC14-XHT82
Accreditation Hours
2.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-23-014-H01-P/T will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Pamela Sardo, PharmD, BS
Freelance Medical Writer
Sardo Solutions
Josephine, TX
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Dr. Sardo worked for Rhythm Pharma until March 2022. We identified no potential financial or other conflicts of interest.
ABSTRACT
Ulcerative colitis is an idiopathic relapsing/remitting disease that is increasing in incidence and prevalence. It is characterized by inflammation, abdominal cramping, bloody diarrhea, fatigue, and bowel urgency among other symptoms. It often presents in teens and young adults with various degrees of severity and significantly impacts quality of life. Primary treatment objectives include achieving rapid resolution of symptoms, mucosal healing, clinical and endoscopic remission, and improving a patient’s quality of life. In moderate to severe disease, prescribers employ immunosuppressive medications, anti-TNF agents, IL 12/23 antagonists, adhesion molecule inhibitors, JAK inhibitors, and S1P receptor modulators. Two organizations have published joint recommendations regarding these pharmaceuticals’ place in therapy. As more data is published and investigational therapies are approved, the treatment approach will continue to evolve. Ulcerative colitis is incurable, so some patients may require surgery. Optimizing care with a multidisciplinary team, including pharmacy personnel, remains an opportunity in this complex condition.
CONTENT
Content
INTRODUCTION
Fiery inflammation in the bowel can be due to several conditions. In inflammatory bowel disease (IBD), gastrointestinal (GI) tract inflammation episodes are common. Crohn’s disease (CD) and ulcerative colitis (UC) are two IBDs differentiated by location and bowel involvement.1 That’s a point to remember: that IBD is an umbrella term that includes CD and UC. In 10% to 15% of patients, the features of CD and UC are so similar that it is impossible to differentiate between them and misdiagnosis may result.1,2 Besides the GI tract, both CD and UC also may be accompanied by symptoms outside the intestine, called extraintestinal symptoms.
DIFFERENTIATING CD AND UC
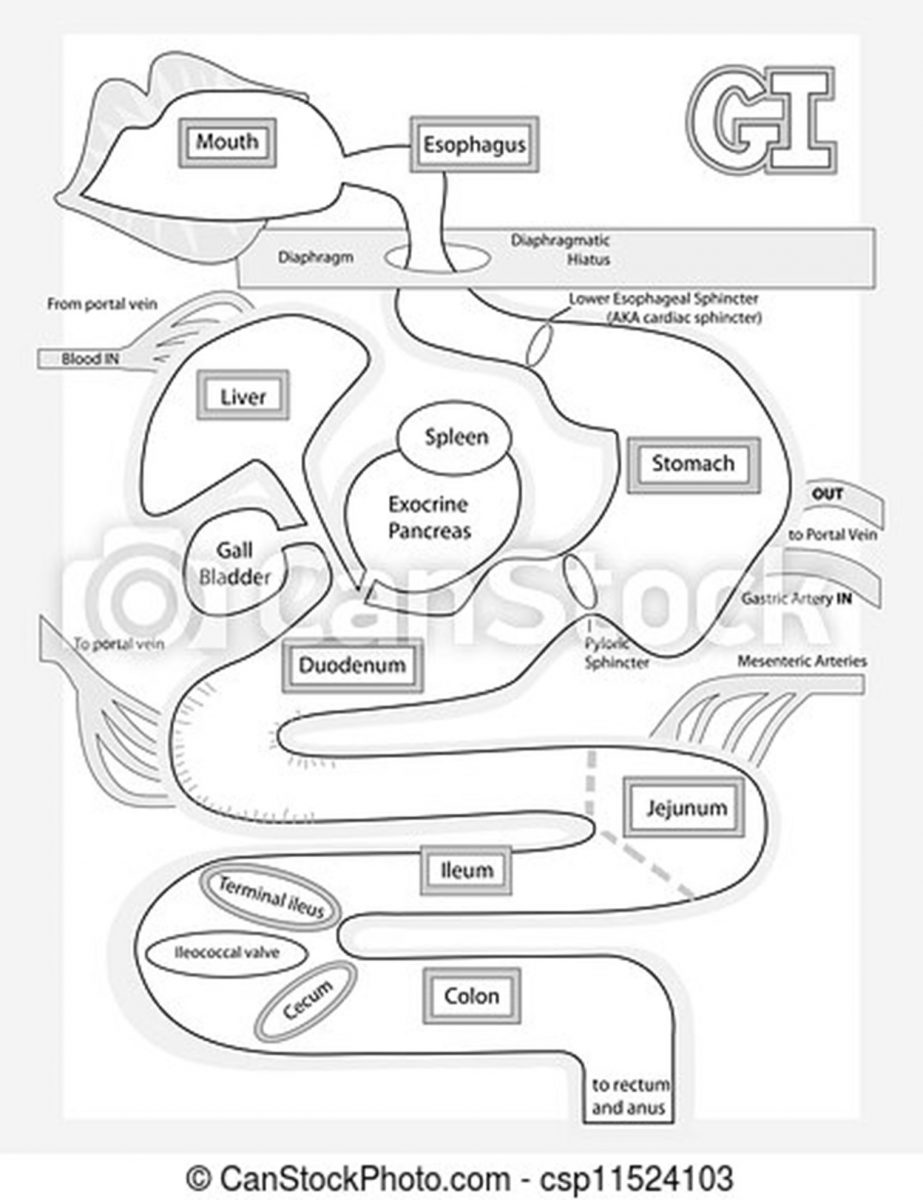
CD results in patchy ulceration of any portion of the GI tract (see Figure 1) from the mouth to the anus. It frequently affects the terminal ileum and colon, and bleeding is uncommon. Typically, patients experience pain in the lower right abdomen.3 UC, this continuing education’s primary focus, involves inflammation of the colon mucosa. UC often affects the rectum, referred to as proctitis, and bleeding during bowel movements is common. Typically, patients experience pain in the lower left abdomen. It may extend into the left (sigmoid) part of the pelvis or beyond the sigmoid, or include the entire colon, referred to as pancolitis.4 In UC, inflammation leads to edema, ulcers, bleeding, and electrolyte losses.
Figure 1. The Gastrointestinal Tract
UC is a chronic idiopathic relapsing/remitting condition (meaning it waxes and wanes) with interactions between the environment, immune system, gut microbiome, and a genetic predisposition to the disease suspected as causes.5 UC’s incidence and prevalence is increasing worldwide.6 From 1990 to 2017, its incidence increased from 3.7 million individuals to 6.8 million affected individuals worldwide.7 UC is incurable, so treatment goals aim to achieve rapid resolution of symptoms, mucosal healing, and clinical and endoscopic remission. An additional goal is to improve a patient’s quality of life (QoL).6.
Individuals with this complex condition have a high level of disability and high healthcare resource utilization. Beyond medical management, 15% to 20% of patients with UC will require surgery. Compared to adults without IBD, adults with IBD experienced $11,029 higher direct costs per patient per year according to one report.8 Documenting this diagnosis in the pharmacy’s profile can help pharmacists and technicians screen for potential problems more efficiently.
Differentiating the two forms of IBD drives treatment. Both CD and UC present as similar repetitive episodes of GI inflammation and create significant patient burden. In CD, inflammation or ulceration commonly occurs in the ileum and colon.4 Outside of the intestine, CD may affect the esophagus, duodenum, or stomach, and gallstones may occur. In UC, extraintestinal manifestations may appear most commonly as inflammatory arthropathies (joint disease) and bile duct inflammation and scarring, but may include bone, eye, and skin involvement.9,10 Although IBD’s incidence peaks at age 15 to 29 years, 10% to 15% of new diagnoses occur among adults aged 60 years or older.11 Both forms of IBD are more prevalent among non-Hispanic White people than among people in other racial/ethnic groups.12
PAUSE AND PONDER: What support can you provide to a patient who tells you that wherever they go, their first action is to scan for the closest bathroom?
UC’S PATHOPHYSIOLOGY AND ASSESSMENT
The normal colon is five or six feet long and three inches wide. Its layers of circular and longitudinal muscles and tissues contract to move food and liquid forward. It wraps around the outside of the small intestine, has segments, and looks somewhat flat. A seam runs vertically down the middle so the segments bulge on either side of the seam. As UC becomes chronic, the colon becomes more rigid and short, leading to a pipe-like appearance.9
The most important risk factor for UC is a family history of IBD in a first-degree relative.14 Patients with disease extension to the left side of the colon, or extensive colitis, are more likely to need medication, undergo colectomy (removal of a portion of the colon), and develop colorectal cancer.15 Risk factors for colorectal dysplasia (abnormal that are not cancerous but can sometimes lead to cancer) or cancer include disease duration and extent, active inflammation, presence of a stricture (inelastic narrowing of a section of the GI tract), polyps, and family history of colorectal cancer. The bile duct may also become diseased in 3% to 7% of patients with UC.15,16
UC’s pathophysiology involves defects in the epithelial barrier, defective immune response, the presence of leukocytes, and microflora imbalance in the colon.17,18 UC’s inflammation deteriorates the epithelium, and exposure to certain intestinal microbes worsens the inflammation.9 Other immune-related factors that affect UC’s pathophysiology and the body’s response include tumor necrosis factor-alpha (TNF-alpha), numerous interleukins, and elevated immune globulin levels.17
Two measures are recognized in UC. Gastroenterologists and researchers tend to use the erythrocyte sedimentation rate (ESR) and c-reactive protein (CRP) components of the Truelove and Witts criteria. Researchers frequently measure ESR and CRP before and during treatments as outcome measures. The prescribing information for anti-TNFs, JAK inhibitors, and IL-12/23 antagonists also include ESR and CRP measures. Table 1 illustrates UC disease severity measured by assessment in the modified Truelove and Witts criteria.15
Table 1. Modified Truelove and Witts Criteria15
| Parameter | Mild | Moderate | Severe |
| Bloody stools/day (n) | <4 | 4-6 | >6 |
| Pulse (beats/minute) | <90 | ≤90 | >90 |
| Temperature (T) °C
(T°F ) |
<37.5
(<99.5) |
37.5 – 37.8
(99.5 – 100.4) |
>37.8
(>100.4) |
| Hemoglobin (g/dL) | >11.5 | 11.5 – 10.5 | <10.5 |
| ESR (mm/hr)
[or CRP mg/l] |
<20 (normal) | 20 – 30 (<30) | >30 (>30) |
°C=degree Celsius; CRP = c-reactive protein; ESR = erythrocyte sedimentation rate; °F = degree Fahrenheit
In the clinical setting, the simpler Mayo score (see Table 2) is used more widely than Truelove and Witts criteria.19 It reflects stool frequency, rectal bleeding, a physician’s global assessment, and a measure of mucosal inflammation at endoscopy, with a maximum score of 12. Clinical response in UC is defined as19
- a reduction of Mayo score by at least 3 points and a decrease of 30% from the baseline score, or
- a decrease of at least 1 point on the rectal bleeding subscale or
- a total rectal bleeding score of 0 or 1
Table 2. Mayo Score for Ulcerative Colitis15,20,19
| Points | ||||
| Mayo Variables | 0 | 1 | 2 | 3 |
| Stool frequency | Normal | 1-2/day more than normal | 3-4/day more than normal | 5/day more than normal |
| Rectal bleeding | None | Streaks of blood with stool <50% of the time | Obvious blood with stool most of the time | Blood passed without stool |
| Mucosa (endoscopic subscore) | Normal or inactive disease | Mild disease (erythema, decreased vascular pattern, mild friability) | Moderate disease (marked erythema, lack of vascular pattern, friability, erosions) | Severe disease (spontaneous bleeding, ulcerations) |
| Physician’s global assessment | Normal | Mild disease | Moderate disease | Severe disease |
Mayo score = sum of scores for each of the four variables (maximum score 12)
Beyond clinical response, clinical remission is a desired objective and is defined as a Mayo score of 2 or less and no individual subscore exceeding 1.19 Mucosal healing is defined as a mucosa subscore of 1 or less. Disease activity is a measure used to decide what treatments to prescribe. Mild disease activity is defined as scores of 3 to 5. Moderate and severe disease activity are defined as scores between 6 and 10 and 11 and 12, respectively.19
The clinician’s patient assessment must be global (all encompassing).21 During patient interviews, clinicians should inquire about extraintestinal symptoms, which may include joint, mood, ocular, oral, or skin changes. They should order laboratory evaluation for anemia and liver function abnormalities. Clinicians should explore QoL issues, such as impact on school, work, or personal relationships. Treatment plans should incorporate patients’ unmet needs and preferences. Exploring the need for social and emotional support, financial resources, and adequacy of patient education regarding their disease are important.21
Throughout treatment, it’s important to assess the patient’s clinical response and remission periodically. Clinicians should monitor cross-sectional imaging, specifically MRI, CT and ultrasound, and surrogate markers, such as fecal calprotectin (FCP; type of white blood cell that migrates to inflamed tissue) and CRP.15,22 A sigmoidoscopy or colonoscopy is recommended within three to six months of initiating treatment to determine the level of suppression of the inflammation, treatment response, or the need to modify the treatment.22
THE PATIENT’S JOURNEY
The journey through symptoms, diagnosis, and treatment to quell the fiery inflammation associated with UC can be long and difficult. In one study, one in four individuals with IBD reported GI symptoms to their primary care physician more than six months before receiving a diagnosis.23 Of these individuals, 10.4% reported symptoms five years before receiving a UC diagnosis. Delayed diagnosis often results in disease progression, worsening tissue, and mucosal damage, surgery, colectomy, or colitis-associated cancer. Patients with a previous diagnosis of irritable bowel syndrome or depression were less likely to receive a timely specialist appointment.23
UC is vastly heterogeneous. Table 3 presents two cases with distinct disease journeys.
Table 3. Two Distinct Patient Journeys24,25
| UC Patient Case 1 | UC Patient Case 2 | |
| 9-year-old girl diagnosed after ileocolonoscopy reveals diffuse moderate inflammation | UC since age 18 | |
| Moderate clinical disease activity, Mayo score = 8 | Symptoms included bleeding, severe pain and swelling in lower abdomen, gas, and nausea | |
| Low hemoglobin, low vitamin D | Lifestyle: Undergraduate, lifeguard, swim coach | |
| Oral corticosteroids started followed by dose tapering dose and mesalamine started | Daily regimen of oral medication, rectal suppositories, nightly enemas | |
| At week 4, active disease remained | Symptoms worsened so → ED | |
| IFX started at 10 mg/kg at 0, 2, and 6 weeks | Barely able to eat or drink, covered with rash and inflamed eyes | |
| At 26 weeks with IFX, she remained in clinical remission | Incontinence in street, bus, and post office | |
| At 1 year, she remained in remission | Acute diarrhea with each BM → became homebound | |
| Follow-up flexible sigmoidoscopy demonstrated mucosal healing | At age 29, married → then 10 weeks of hospitalization without remission | |
| Agreed to surgical colectomy → difficult recovery, diarrhea, and intestinal blockages | ||
| Finally exercising, back to work, and monitoring the quantity, texture, and timing of food. Remained on pre-surgical medications. |
BM = bowel movement; ED = emergency department; IFX = inFLIXimab
UC affects many distinct populations. In the pediatric population with UC, unlike in adults, the clinical condition can present atypically with a more severe, early onset and continuous inflammation of the rectum and colon.26,27,28 In these patients, clinicians should assess disease location and severity. One study indicated 62% of pediatric patients with UC from birth to age 5 years had extensive pancolitis, and 38% and 31% from ages 6 to 11 and from ages 12 to 18, respectively, had pancolitis.27 Children can also experience rectal sparing (a normal/unaffected rectum) and limited distal disease.26,28
To optimize management, enhance QoL, and minimize complications in all patients, continued patient engagement is important.29
GOALS OF UC MANAGEMENT
The UC treatment guidelines recommend goals of therapy to include29
- induction and maintenance of clinical and endoscopic remission
- maintaining steroid-free remission
- improving QoL, and
- preventing complications, hospitalizations and surgery.
Pursuing these goals can also contribute to minimizing cancer risk.21,30,31 Long-term mucosal healing may reduce the risk of dysplasia.31
The Treat-To-Target Approach
UC is considered relapsing/remitting because it presents as relapses of symptoms and then periods of symptomless response and remission. Beyond symptom remission, a treat-to-target (T2T) approach focuses on32,33
- minimizing disease activity
- reducing futures risks and
- reducing future relapses or complications such as ileal strictures (narrowing), fistulas, functional impairment, or colon cancer.
In 2015, the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative recommended that UC treatment goals should address two targets: clinical and endoscopic outcomes.32 IBD experts recommend using measures of inflammation such as FCP.32 FCP detects GI inflammation sensitively and is used to monitor disease activity and predict relapse.
PAUSE AND PONDER: Which UC treatments do you see in your practice location most often?
UC’s treatment options are plentiful and evolving. Pharmacy team members can collaborate with multidisciplinary professionals regarding patient care for UC. (See SIDEBAR). Pharmacists’ drug therapy and disease management expertise can promote individualized treatment decisions for these complex patients.
SIDEBAR: Working with the Multidisciplinary Team
Team collaboration can increase patient and provider education, enhance quality of care, and reduce disease burden, and morbidity. Many pharmacists don’t know how to introduce themselves to or work with multidisciplinary teams. Sometimes, team members from other disciplines will call with questions or concerns, and the call opens the door for regular communication. Often, however, pharmacy staff will need to take the initiative and introduce themselves. Here are four tips to working better with the UC care team.
- Ask patients if they are seeing a nutritionist, primary care provider, gastroenterologist, or behavioral health provider (for stress support). If UC is severe, inquire if they have a surgeon or know their radiologist. Record these providers’ names in the patient record and contact them when questions or concerns arise.
- Make the patient your ally and be your patient’s ally. When you educate patients well and show them your capabilities, they will report back to their clinicians.
- When you work with a patient who has UC, let the professional team members know. Sending a quick note to say that, for example, the patient’s adherence was poor and you discussed ways to improve it with the patient sheds light on possible non-response. It also makes clinicians aware that you have skills and you’re not afraid to use them!
- Don’t be afraid to make team members aware of issues like cost or availability of less expensive options. Often, prescribers have no idea that patients experience sticker shock when they fill their prescriptions.
TREATMENT
Medication is a mainstay of treatment for patients with UC. Prescribers may combine, dose-escalate, reduce, or discontinue medications, depending on the patient’s disease severity.
Traditional Treatments
When a patient presents with mild to moderate UC, the expert consensus is to start treatment with an oral 5-aminosalicylate (5-ASA; sulfasalazine, mesalamine, and diazo-bonded 5-ASA [the prodrugs, balsalazide and olsalazine, which convert to mesalamine]34) with or without a rectal 5-ASA.34,35 In many cases, rectal dosing improves symptoms; using a suppository, enema, or rectal foam applies the medication exactly where needed. Sulfasalazine, balsalazide, and mesalamine are similarly effective and safe.34 Some patients will respond inadequately and may need to escalate therapy to systemic corticosteroids, immunomodulators (IMM), biologics, or other agents. If symptoms persist, it is important to rule out infection, optimize adherence, increase the oral dose, and add rectal 5-ASA if not yet prescribed.34,35
Comparative efficacy studies are helpful to clinicians. One technical review of multiple drugs in mild to moderate UC compared rectal 5-ASAs (5-ASA enemas 1 to 4 g/day or 5-ASA suppositories 1g/day) to rectal corticosteroids (hydrocortisone enema 100mg/day, prednisolone enema 25–30 mg/day, budesonide enema 2 mg/day, beclomethasone 3 mg/day or comparable foam) in 13 trials.36 In mild to moderate ulcerative proctosigmoiditis cases treated for two to eight weeks, rectal 5-ASAs were superior to rectal corticosteroids for induction of clinical remission.36
Table 4 lists the broad categories of medications for mild, moderate, or severe UC and dosing information.
Table 4. Medications for UC22,37-42
| Category | Substance | Dosage |
| 5 – ASA | Mesalamine
Balsalazide
Olsalazine
Sulfasalazine |
2 – 4.8 g/day (oral)
1 – 2 g/day (rectal)
6.75 g/day (rectal)
1 g/day (oral)
2 – 4 g/day |
| Corticosteroids | Budesonide
Budesonide MMX
Prednisone
Hydrocortisone
Methylprednisolone |
2 mg/day (rectal)
9 mg/day (oral)
0.75 – 1 mg/kg/day
100 mg IV 4 times/day
125 mg IV/day |
| Thiopurines
Immunosuppressives |
Azathioprine
6-mercaptopurine |
2 – 2.5 (max 3) mg/kg/day
1 – 1.5 mg/kg/day |
| Calcineurin inhibitors | Cyclosporine
Tacrolimus |
2 mg/kg/day IV
0.2 mg/kg/day |
| Anti-TNF agents | Adalimumab
Golimumab
Infliximab |
160 mg wk 0, 80 mg wk 2, 40 mg wk 4, then 40 mg every 2 wks; may ↑ to 40 mg/wk SUBQ
200 mg wk 0, 100 mg wk 2, 50 mg wk 4, then 50 mg every 4 wks; may ↑ to 100 mg if pt>80 kg SUBQ
5 mg/kg wk 0, 2, 6, then every 8 wks IV |
| Adhesion molecule inhibitors
(anti-integrin) |
Vedolizumab | 300 mg wk 0, 2, 6, then every 8 wks IV |
| Janus kinase inhibitor | Tofacitinib
Upadacitinib |
5 – 10 mg/day (oral)
First 8 wks: 10 mg twice/day 10 mg twice/day for 8 more wks if partial response Then 5 mg twice/day or 22 mg XR/day for 8 weeks; then evaluate
45 mg/day for 8 wks then 15 mg/day (oral) |
| Interleukin 12/23 antagonist | Ustekinumab | 250 mg to 55 kg IV
390 mg if >55 kg – 85 kg IV 520 mg if >85 kg IV Then 90 mg SUBQ every 8 wks |
| Sphingosine 1-phosphate receptor modulator | Ozanimod | 0.23 mg days 1-4, 0.46 mg days 5-7, then
0.92 mg/day (oral) |
G = grams; IV= intravenous; kg=kilogram; mg= milligrams; MMX = Multi-Matrix System technology drug release; SUBQ=subcutaneous; wk= week
Sulfasalazine
If sulfasalazine is prescribed, 4 g provides approximately 1.6 g of 5-ASA equivalence.34 Typical doses are 2 tablets 4 times a day.37,43 In some cases, prescribers may initiate therapy with a smaller dose, (e.g., 1 to 2 g daily) to reduce possible GI intolerance and slowly increase as tolerated. They may also try enteric-coated sulfsalazine.37,43 Sulfasalazine is commonly prescribed for individuals with UC and rheumatologic comorbidities.43 Periodic monitoring of complete blood counts (CBC) and liver function tests (LFT) is recommended.
Sulfasalazine may be poorly tolerated due to adverse effects such as headache, nausea, diarrhea, and rash.43 Additional adverse events with sulfasalazine include folate metabolism interference, male infertility, and rare cutaneous side effects such as Stevens-Johnson syndrome. Anemia, leukopenia, or thrombocytopenia are also possible. Pneumonitis and hepatitis have been reported.43 Sulfasalazine is contraindicated if a patient has a sulfa allergy. Sulfasalazine tablets are a yellow/orange color; individuals who take it may notice an orange tinge in urine, tears, and sweat that can stain clothing and contact lenses. Auxiliary labels and counseling should emphasize drinking plenty of fluids, taking the drug on an empty stomach, and avoiding antacids.
Mesalamine and Balsalazide
Low dose mesalamine may be prescribed initially at less than 2 grams per day(g/d). Some patients need 2 to 3 g/d. Higher doses of 3 g/d or more may be required to induce remission.36 Combining oral and rectal therapy may deliver a higher effective dose of 5-ASA to the involved area and lead to higher rates of induction and maintenance of remission. This approach may avoid escalation to corticosteroids or other agents.36
Mesalamine and balsalazide are associated with rare idiosyncratic worsening of colitis.22 Rare interstitial nephritis may also occur. The prescribing information recommends periodic renal function monitoring. Olsalazine is generally less well tolerated than either mesalamine or balsalazide. Headache, nausea, diarrhea, leukopenia and hepatitis are possible. 5-ASA treatments should be avoided in pregnancy.22
Steroids and Other Traditional Treatments
Corticosteroids, such as budesonide, are generally prescribed for a short duration or for induction of remission.34 Some experts suggest prescribing oral mesalamine, balsalazide or olsalazine over budesonide because hepatic first-pass metabolism lowers budesonide’s systemic activity.34,44 As the patient’s condition improves, withdrawing the steroids and reaching steroid-free remission (SFR) is important. Long-term corticosteroid use may lead to infection, diabetes mellitus, weight gain, insomnia, osteoporosis, or glaucoma.45 Mood changes, cataracts and delayed wound healing are possible.45
Thiopurines and Cyclosporine
Thiopurines, including azathioprine and 6-mercaptopurine, may improve inflammation in patients with UC. They inhibit the proliferation of lymphocytes and are used to maintain remission. Their dosing is weight-based and their onset of action can be slow, taking up to three months.22 They are often used in combination with biologics. Monitoring for drug interactions is necessary. Allopurinol interactions with thiopurines are especially important because allopurinol inhibits thiopurine metabolism.46 The dose of mercaptopurine or azathioprine may need to be reduced to one-third or one-quarter of the normal dose if allopurinol is also prescribed. Prescribers determine dose reductions based on therapeutic response and toxicity.47
Even though thiopurines and cyclosporine have been prescribed for many years, remaining aware of their side effects is important. Possible adverse events of thiopurines include nausea, vomiting, fever, leukopenia, thrombocytopenia, pancreatitis, and hepatotoxicity. Rare adverse events may include non-Hodgkin lymphoma. Individuals prescribed calcineurin inhibitors, such as cyclosporine, may experience hypertension, nephrotoxicity, hyperkalemia, infection, lymphoma, or diabetes mellitus.37
Additional therapies include biologics and newer small molecules (tofacitinib, ozanimod). Before discussing newer therapies, a short review of step-up and step-down approaches is reasonable. If a patient presents with moderate to severe ulcerative colitis, philosophies differ among gastroenterologists regarding beginning with a step-up versus a step-down approach.36,48
- The step-up approach begins with the traditional therapies mentioned above and followed by biologics and IMM if symptoms persist, if remission is not achieved, and the patient is considered high-risk. Extensive inflammation and the need for repetitive use of corticosteroids are examples of high-risk status.
- The step-down approach occurs when prescribers begin with a more intensive treatment with biologics and IMM and then step down to the medications mentioned above if patients improve.
Step Up or Step Down?
A 2021 study (N = 1891) examined UC’s clinical presentation in the five years before starting biologic therapy. The researchers analyzed patients’ experiences, comorbidities, morbidity, and treatment burden over time. Figure 2 summarizes participants’ health burden, disease progression, medication burden, and increased comorbidities. Across the study period, the need for treatment with oral corticosteroids, 5-ASA, and other non-biologic immunomodulators (IMM) increased progressively. Due to the increasing burden, the researchers supported early use of biologics (a step-down approach) rather than gradual step-up after failing conventional therapies for adult patients with moderate to severe UC.30
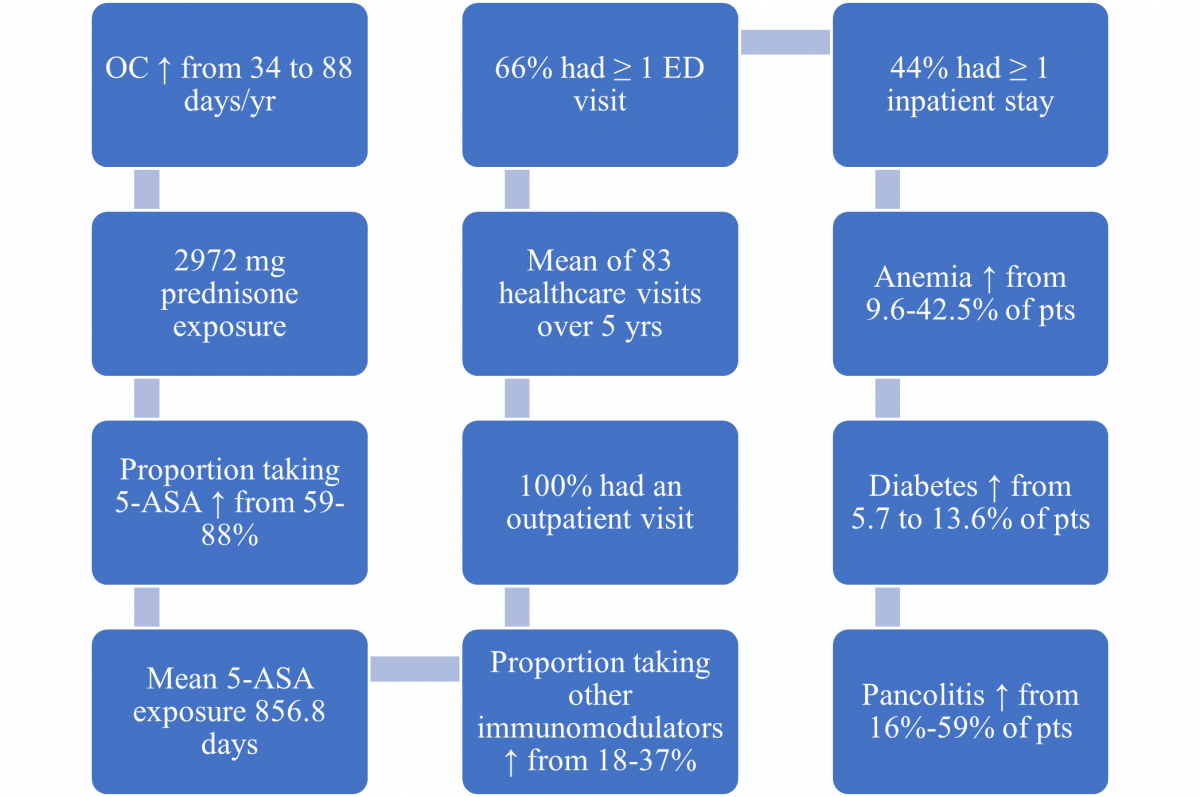
Figure 2. Treatment and Health Experience Beginning Five Years Before First Biologic30
5-ASA = 5-aminosalicylates; ED = emergency department; OC = oral corticosteroids; Pts = patients
Anti-TNF agents
Anti-TNF agents are commonly prescribed for moderate to severe UC due to their effectiveness, length of time on the market, and healthcare professional comfort prescribing them.44 The U.S. Food and Drug Administration (FDA) has approved many anti-TNFs, but has approved only adalimumab, golimumab, or inFLIXimab for treating UC. Usually, prescribers use anti-TNFs after 5-ASA and corticosteroids in patients with mild to moderate severity and the step-up approach to treatment. Prescribers may prescribe the step-down approach, using biologics earlier, in some cases.38 They often employ concomitant IMM to decrease immunogenicity seen with anti-TNFs.38,47
Because biologics like anti-TNFs are made with living cells, patients may develop an unintended immune response (immunogenicity) in the form of anti-drug antibodies; if they develop, these antibodies may render the anti-TNF medication less effective. The antibodies may bind to the anti-TNF, interfere with its mechanism of action, reduce the anti-TNF’s serum concentration, increase its clearance, and lead to loss of effectiveness. Antibodies may also occur upon restarting an anti-TNF if a patient starts and stops an anti-TNF over time. It is unknown why some people develop anti-drug antibodies and others do not. The presence of antibodies and reduced response often prompt prescribers to switch the anti-TNF to regain effectiveness, and doing so often works. The addition of an IMM can suppress the antibodies which may reduce the risk of switching therapies or avoiding an anti-TNF dose increase.47
Pharmacy team members should remain diligent about look-alike, and sound-alike names of the anti-TNF agents and should remember that doses of anti-TNF agents vary by indication.
SIDEBAR: TECH TALK about Look-alike, Sound-alike medications
Medications with names that look-alike and/or sound alike are classified as high-alert medications.
- Know that inFLIXimab had been confused with riTUXximab, so the Institute for Safe Medication Practices recommends using TALL Man lettering.
- Prevent medication errors between therapies for UC, such as adalimumab and golimumab, ustekinumab and upadacitinib, or vedolizumab and ustekinumab or adalimumab. Segregate the medications in different locations or consider stickers or other alert methods prior to medication dispensing.
An Anti-integrin
Integrins are adhesion receptors that mediate cell-to-cell and cell-to-extracellular adhesion. Vedolizumab is a humanized monoclonal antibody that binds to the α4β7 integrin, blocks the interaction of α4β7 integrin, and inhibits lymphocyte migration into inflamed GI tissue.49 With fewer lymphocytes in the GI tract, vedolizumab helps reduce inflammation and UC symptoms. Vedolizumab can be referred to as a B-anti-integrin and an adhesion molecule inhibitor. Patients with steroid-dependent disease have treatment options, including treatment with thiopurines, anti-TNF agents (may be combined with azathioprine or 6-mercaptopurine), or vedolizumab.38
Prescribers and patients may ask about outcomes in clinical trials. A vedolizumab study enrolled patients with an inadequate response or intolerance to IMM therapy (i.e., azathioprine or 6-mercaptopurine) and/or an inadequate response, loss of response, or intolerance to an anti-TNF.49 Participants received IV vedolizumab 300 mg or placebo at week 0 and week 2. Concomitant stable dosages of 5-ASA, corticosteroids (prednisone dose of 30 mg/day or less or equivalent), and IMM (azathioprine or 6-mercaptopurine) were permitted through week 6. The percentage of patients achieving a clinical response at week 6 was 47%. The researchers also measured remission and found 17% of participants achieved remission at week 6. Those with a clinical response at week 6, or receiving open-label vedolizumab, were invited to enter a 52-week study with every 8-week dosing. At week 52, 42% of participants achieved clinical remission.49
JAK inhibitors
Tofacitinib and upadacitinib are referred to as small molecules because their molecular structure is smaller and simpler than the biologics’, which are large three-dimensional structures. The JAK inhibitors are dosed orally and require a loading dose. JAK inhibitors are indicated for adults with moderately to severely active UC who responded inadequately or couldn’t tolerate one or more anti-TNF agents.39 Many individuals are willing to visit an infusion suite for treatment; however, younger patients and those with very busy lifestyles or heavy travel schedules may prefer the small molecule oral drugs for their convenience.
When prescribing JAK inhibitors, pretreatment screening should include assessment for hepatitis B and tuberculosis. When patients start biologics or JAK inhibitors, their immune response may decrease, increasing risk of certain infections, such as hepatitis B or tuberculosis. If patients have active hepatitis B or tuberculosis, they need treatment to eradicate those infections before beginning biologics or JAK inhibitors. A risk of bacterial, viral, and herpes zoster infections exists, so prescribers must assess vaccination status before treatment. CBC and lipid panel monitoring is recommended. Prescribing tofacitinib in combination with biologics for UC or with potent IMM, such as azathioprine and cyclosporine, is not recommended.40
IL-12/23 antagonist
Interleukin 12 (IL-12) and IL-23 are proteins that can cause inflammation. Ustekinumab is a human monoclonal antibody against the shared p40 subunit of IL-12 and IL-23 cytokines. Ustekinumab disrupts inflammation by blocking IL-12/23.41 The FDA has approved this biologic for adults with various forms of psoriasis, CD, and UC. (It is also approved for children 6 and older for various forms of psoriasis.) In UC in adults, it is typically administered as an IV induction dose, followed by subcutaneous maintenance dosing every eight or 12 weeks. Clinicians should screen for hepatitis B and tuberculosis and monitor CBC every six months.41 To minimize the risk of medication errors, pharmacy staff should double-check the prescribed route (IV or subcutaneous). They should also be alert for look-alike, sound-alike issues, to prevent prescription transcribing errors or other confusion between ustekinumab and the JAK inhibitor upadacitinib.
Research has documented outcomes for this monoclonal antibody, too. A ustekinumab clinical trial assessed efficacy and safety in adults with UC; the primary endpoint was clinical remission at week 8.41 The researchers randomized participants to a single IV dose of ustekinumab of approximately 6 mg/kg, 130 mg (a lower dose than recommended), or placebo at week 0. Enrollment required previous inadequate response to corticosteroids, IMM, or at least one biologic. At week 8, 19% of participants successfully reached clinical remission.41
S1P Modulator
Ozanimod is an oral sphingosine 1-phosphate receptor (S1P) modulator indicated for the treatment of moderately to severely active UC in adults.42 It is also approved for relapsing forms of multiple sclerosis, and the pharmacy team may field questions about this medication’s various indications. Ozanimod binds with high affinity to S1P receptors 1 and 5. It blocks lymphocyte trafficking from lymph nodes, reducing the number of lymphocytes in peripheral blood. Ozanimod may transiently decrease heart rate and cause atrioventricular conduction delays, so a low-dose starter titration pack is available. Prescribers should escalate the dosing slowly to avoid rare but significant bradycardia. Progressive multifocal leukoencephalopathy, a disabling, sometimes deadly adverse event, is rare but possible. Pharmacy teams should counsel patients regarding the need for effective contraception to prevent pregnancy; patients should use contraceptives for three months after discontinuing ozanimod.42
An ozanimod clinical trial assessed efficacy and safety using a primary study endpoint of clinical remission at week 10.42 It enrolled adults with moderately to severely active UC if they had an inadequate response, or were intolerant, to specific other treatments. The treatments included oral 5-ASA, corticosteroids, IMM (e.g., 6-mercaptopurine and azathioprine), or a biologic (e.g., anti-TNF and/or vedolizumab). The proportion of patients reaching clinical remission at week 10 was 18% with an ozanimod dose of 0.92 mg once daily. Those achieving a clinical response were invited to be re-randomized to a 52-week study extension. The primary endpoint was the proportion of patients in clinical remission at week 52. The proportion of patients with an ozanimod dose of 0.92 mg once daily with clinical remission at week 52 was 37%.42
MEDICATIONS IN DEVELOPMENT
Etrasimod is a once-daily, oral S1P receptor modulator in development for the treatment of UC. Results from the phase 2 OASIS trial and open-label extension study revealed patients with moderately to severely active UC who received etrasimod showed improvements in clinical remission and symptom relief beginning as early as week 2. In the phase 3 ELEVATE UC 52 study, 27% of patients in the etrasimod group achieved clinical remission compared with 7% of patients in the placebo group at 12 weeks.50 Use of etrasimod in UC is not FDA-approved and regulatory authorities have not yet evaluated its safety and efficacy.
Risankizumab-rzaa is an IL-23 inhibitor that is FDA-approved for psoriasis and Crohn’s disease. It is in development for treatment of individuals with UC. In the INSPIRE trial, 20.3% of participants with intolerance or inadequate response to conventional or advanced therapies (biologics, JAK inhibitors, and S1P receptor modulators) achieved clinical remission at week 12.51 The FDA has not approved risankizumab in UC or evaluated its safety and efficacy.
Considerations for Medications in Therapy
Decision-making regarding UC treatment requires consideration of many factors, including
- disease and inflammation location, severity, and extent
- comparative effectiveness and long-term safety of available treatments
- treatment availability
- product labeling
- guideline recommendation
- prior treatment successes or failures
- cost, and
- patient preferences
Since some prescribers may dose escalate to an off-label (higher) dose or off-label shorter dosing interval, pharmacy teams need to remain alert regarding insurance policies that may impact treatment decisions.
Safety Information
Each medication’s full prescribing information includes comprehensive safety and efficacy details. The information in this section is not all-inclusive. It’s critical to keep in mind that all UC treatments have limitations. Because most infusions have similar adverse events, Table 5 summarizes select safety information and limitations regarding newer agents used in UC.
Table 5. Newer Agents: Select Safety Information and Limitations 37,41,42,49,52
| Limitations and Safety Considerations | Anti-TNF agents | Anti-IL-12/23
Agents |
JAK Inhibitors | Anti-integrin | S1PR
modulator |
| Immuno-suppression | √ | √ | √ | √ | √ |
| Infection | √ | √ | √
(herpes zoster) |
√
(upper respiratory) |
√ |
| Venous thrombo-embolism | √ | ||||
| Psoriasis | √ | √ | |||
| Major CV adverse event | √ | √ | |||
| Infusion/ injection site reaction | √ | √ | √ | √ | |
| Malignancy | √ | √ | √ | √ | √ |
| Tuberculosis | √ | √ | √ | √ | |
| Worsen CHF | √ | ||||
| Lymphoma | √
(if combine with thiopurines) |
√ | √ | ||
| Lymphocyte abnormalities | √ | ||||
| Anemia | √ | √ | |||
| Elevated lipids | √ | ||||
| Headache | √ | √ | √ | √ | √ |
| Nausea | √ | √ | √ | √ | |
| Fatigue | √ | √ | √ | √ | √ |
| Liver function test elevations | √ | √ | √ | ||
| Contra-indication if post-MI within 6 months, or unstable angina, stroke or TIA | √ | ||||
| Contraindicated if severe untreated sleep apnea | √ | ||||
| PML | √ | √ |
MI = myocardial infarction; PML = progressive multifocal leukoencephalopathy; TIA = transient ischemic attack
Identifying drug interactions is a key skill for pharmacists. Of note, JAK inhibitors can interact with CYP3A4 inhibitors (e.g., ketoconazole, clarithromycin) and CYP2C19 inhibitors (e.g., fluconazole, omeprazole).40 Drug interactions may be a concern with the use of ozanimod and concurrent IMM, tyramine, antiarrhythmics, beta blockers, or calcium channel blockers.42
ACG and AGA Guidelines for the Management of Moderate-to-Severe UC
The American College of Gastroenterology (ACG) and the American Gastroenterological Association (AGA) published comprehensive treatment guidelines and interventions for UC.31,44 Both guidelines cover achieving a response, induction of remission, and maintenance of remission. Select highlights presented below are not all-inclusive.
For remission induction, ACG recommends the following options31:
- In moderately active UC, oral budesonide MMX
- In moderately to severely active UC, oral corticosteroids
- In moderately to severely active UC, anti-TNF therapy
- When inFLIXimab is used as induction therapy for patients with moderately to severely active UC, it should include a thiopurine
- In moderately to severely active UC, or if failed anti-TNF, vedolizumab
- In moderately to severely active UC, tofacitinib 10 mg orally twice a day for 8 weeks
- For induction of remission in moderately to severely active UC with previous failure of anti-TNF therapy, tofacitinib
For maintenance of remission, ACG recommends the following options31:
- For previously moderately to severely active UC in remission due to corticosteroid induction, thiopurines
- Continue anti-TNF therapy after anti-TNF induction in patients with previously moderately to severely active UC
- Continue vedolizumab in previously moderately to severely active UC now in remission after vedolizumab induction
- Continue tofacitinib in previously moderately to severely active UC now in remission after induction with tofacitinib
The AGA provides the following recommendations for response and remission44:
- Current evidence supports use of inFLIXimab, adalimumab, golimumab, vedolizumab, and tofacitinib for remission induction and maintenance in moderate-severe UC
- Thiopurine monotherapy should not be used for induction of remission but may be considered for remission maintenance
- Meta-analysis suggests that inFLIXimab and vedolizumab may be preferred first-line therapy in biologic-naïve patients, rather than standard-dose adalimumab or golimumab
- In patients with prior inFLIXimab exposure, particularly those with primary non-response to induction therapy, vedolizumab or tofacitinib may be preferred over adalimumab or golimumab
- Combination of a biologic agent with an immunomodulator is more effective than monotherapy with either agent
- In patients with moderate-severe disease activity, at high risk of colectomy, biologic agents with or without an IMM, or tofacitinib, should be used early rather than gradual step-up therapy after failure of 5-ASA
- Patients in remission with biologic agents and/or IMM, or tofacitinib, after prior failure of 5-ASA, may discontinue 5-ASA
PAUSE AND PONDER: What patient support questions may uncover a patient’s adherence challenges?
Patient Education Pearls for Patient Counseling
Pharmacy teams have numerous opportunities to provide UC patient education. Because the condition appears differently in affected individuals, conversations should align with the individual patient’s situation. Supportive patient education pearls addressing inflammation and advancing opportunities to remission should be individualized from these topics53:
- UC’s exact cause is unknown
- UC affects people differs widely
- UC is a chronic condition and symptoms wax and wane
- Medications are available to control UC
- The number of people with UC has been increasing
- It can occur at any age and in any racial or ethnic group
- Symptoms will occur in the intestine and may occur outside of the intestine
- Ulcers in the intestine lining that bleed may lead to low red blood cell count (anemia)
- Ask the doctor what tests are needed
- Diet and nutrition plans differ for each patient
- Managing stress is important
- Have supportive friends and family
- Locate restrooms when outside the home
- Carry extra underclothes, toilet paper or moist wipes
- Ask for school or work accommodations
SIDEBAR: OTC and Alternative Therapies for Patient Discussion34,35,44,54
Patients with UC may use these OTC products:
- Patients with UC may eat less, thinking it will decrease diarrhea. However, they need proteins, water, vitamins, and minerals to promote healing. Patients need vitamin D and calcium for bones if reduce their dairy intake.
- Bismuth subsalicylate (Pepto Bismol) is an antidiarrheal liquid, chewable tablet, and also swallowable tablet. It helps reduce inflammation in the intestinal lining. Remind patients that it darkens stool and the tongue. That darkening is not a medical concern.
- Simethicone (Gas-X) is an anti-flatulent that helps form gas bubbles in the digestive tract, making them easier to pass and relieving gas pain.
- Loperamide (Imodium) should be taken with caution. It slows digestion. Occasional use may be effective, but patients should consult healthcare providers if they contemplate ongoing use.
- If mild disease persists, such as seen with a Mayo score of 1, the AGA guidelines and some advocacy web sites mention curcumin (a phytochemical from turmeric) or suggest adding curcumin and probiotics for some individuals suffering with UC.
- Patients with severe disease, frequent bleeding, anemia, or abnormal laboratory results may need folic acid. The usual folic acid dose is 1 mg/day. Asparagus, broccoli, and spinach are also foods that contain folate or folic acid.
Patients who have UC should avoid these OTC products:
- Patients should consult their healthcare providers whether to avoid aspirin, nonsteroidal anti-flammatories (ibuprofen, naproxen), lactose, sugar substitutes, or preservatives.
- Patients who take sulfasalazine or mesalamine should not take them with antacids.
Many institutions, hospitals and healthcare providers have created UC resources. Table 6 lists examples of supportive options that can be shared with individuals with UC.
Table 6. Patient and Clinician Resources to Support Individuals with UC
| Resource | Contact |
| American College of Gastroenterology | https://gi.org/topics/ulcerative-colitis/
· Describes symptoms, tests, diagnosis, risks, surgery and treatments |
| Cleveland Clinic: Butts and Guts podcast | https://my.clevelandclinic.org/podcasts/butts-and-guts
· Covers a wide range of gastrointestinal issues including management and surgery · Use the search term “ulcerative colitis” |
| Crohn’s and Colitis Foundation (CCF)
|
Help Center (referrals, insurance info)
https://www.crohnscolitisfoundation.org/ info@crohnscolitisfoundation.org 1-888-MY-GUT-PAIN (888-694-8872- extension 8) Signs and Symptoms https://www.crohnscolitisfoundation.org/sites/default/files/legacy/assets/pdfs/living-with-ulcerative.pdf Spanish Help Center School Accommodation Suggestions |
| Mayo Clinic | https://www.mayoclinic.org/diseases-conditions/ulcerative-colitis/symptoms-causes/syc-20353326
· Includes a video and written materials on diagnosis, symptom management, and treatment |
| Downloadable Mobile Apps
· Download from the App Store or Google Play |
My IBD Care: tracks symptoms, flares, medical appointments, BMs, medications
Bathroom Scout: Identifies 1.3 million public toilets MyPlate: Monitors calories, the nutrition content of food MyColitis: Health tracking of bowel movements, medications, moods, symptoms, tests |
CONCLUSION
UC is a debilitating chronic IBD that usually requires long-term treatment to control symptoms and prevent disease-related complications. Many treatments are available; however, the effectiveness, safety, and durability of response and remission vary, and careful assessment of these factors must drive a personalized approach to care. Pharmacy teams must recognize that every patient’s disease is different, and results in diverse manifestations. Pharmacists and pharmacy technicians are ideally positioned to collaborate with multidisciplinary teams to support strategies tailored to each patient to optimize care and to help patients maintain a positive outlook.
Many unanswered questions remain about UC. Researchers will continue to evaluate treatment advances and explore disease management opportunities while pharmacy teams encourage patients suffering from UC to have routine doctor visits, adhere to their medication regimen, and maintain a healthy lifestyle.
Pharmacist Post Test (for viewing only)
Learning Objectives
After completing this continuing education activity, pharmacists will be able to
1. Differentiate UC from Crohn’s disease
2. Describe currently available and novel UC medications under development in the United States
3. Outline the relationship between the types of treatment for UC, patient characteristics and disease severity
4. Identify patient education pearls to address inflammation and advance to remission
1. Which is true regarding the difference between Crohn’s and ulcerative colitis (UC)?
a. Crohn’s disease is limited to inflammation of the colon mucosa
b. Ulcerative colitis can appear anywhere between the mouth and anus
c. Ulcerative colitis involves inflammation of the colon mucosa
2. Which is the usual adalimumab starting dose for a newly diagnosed UC patient?
a. Adalimumab 160 mg at week 0, 80 mg at week 2, 40 mg at week 4
b. Adalimumab 20 mg at week 0, 40 mg at week 2, 50 mg at week 3
c. Adalimumab 200mg at week 0, 90 mg at week 2, 60 mg at week 4
3. Which statement reflects a novel characteristic of ozanimod?
a. Ozanimod is an IL 12/23 monoclonal antibody
b. Ozanimod is an oral S1P modulator
c. Ozanimod is a oral JAK inhibitor
4. When inducing remission, which statement(s) describe(s) ACG recommended UC treatment, patient characteristics, and disease severity?
a. In moderately to severely active UC or if failed anti-TNF, a 5-ASA is recommended
b. In moderately to severely active UC oral corticosteroids may be prescribed
c. In mild-to-moderate UC, lifestyle changes are usually sufficient to induce remission
5. Which are patient education pearls to discuss with a patient suffering with UC?
a. The number of people with UC has been decreasing
b. Intestinal ulcers that bleed will not lead to anemia
c. Many medication options are available to control UC
6. You receive a prescription or order for tofacitinib. Which of the following would be an appropriate dose for a patient who has UC?
a. 5 mg orally once a week
b. 5-10 mg orally daily
c. 10 mg IV every 8 weeks
7. Which statement contains safety information to be aware of with UC treatments?
a. Sulfasalazine carries a risk for venous thromboembolism, herpes zoster, and major cardiovascular adverse events
b. JAK inhibitors may interfere with folate metabolism, affect male fertility, cause rare cutaneous adverse effects
c. Ustekinumab may cause infections, infusion or injection site reactions and may increase the risk of malignancy
8. A 22-year-old college student with newly diagnosed UC is experiencing daily blood in the stool, cramping, and had bowel incontinence on the way to the parking lot after class. Multiple options are available to treat this patient. What are the goals of treatment?
a. To prescribe medication to obtain response in 1 week and remission in 8 days
b. To induce and maintain clinical and endoscopic remission and quality of life
c. To use oral medications only (and avoid infusions) because of her youth and lifestyle
9. A 17-year-old restaurant worker presents with pancolitis. She has no other significant past medical history. She was treated with adalimumab, but the condition is worse with daily blood in the stool, 5 BMs/day, and weight loss of 10 pounds in the past 2 months. She has been reading about UC and is interested in switching to an infusion. Which is a possible IV treatment option?
a. Prednisone
b. Vedolizumab
c. Balsalazide
10. A 30-year-old newly married patient with UC works as a flight attendant. She has pancolitis and was told she is anemic and today has a fever. She comes to the pharmacy before her doctor’s appointment asking which UC medications are oral. Which product names are options to provide this patient?
a. Methylprednisolone
b. Ustekinumab
c. Ozanimod
Pharmacy Technician Post Test (for viewing only)
Pharmacy Technician Learning Objectives
After completing this continuing education activity, the pharmacy technician will be able to
1. Differentiate UC from Crohn’s disease
2. Describe currently available and novel UC medications under development in the United States
3. Outline the relationship between the types of treatment for UC, patient characteristics and disease severity
4. List symptoms that a patient with UC may share with a pharmacy technician
1. Which is true regarding the difference between Crohn’s and ulcerative colitis (UC)?
a. Crohn’s disease is limited to inflammation of the colon mucosa
b. Ulcerative colitis can appear anywhere between the mouth and anus
c. Ulcerative colitis involves inflammation of the colon mucosa
2. Which is the usual adalimumab starting dose for a newly diagnosed UC patient?
a. Adalimumab 160 mg at week 0, 80 mg at week 2, 40 mg at week 4
b. Adalimumab 20 mg at week 0, 40 mg at week 2, 50 mg at week 3
c. Adalimumab 200mg at week 0, 90 mg at week 2, 60 mg at week 4
3. Which statement reflects a novel characteristic of ozanimod?
a. Ozanimod is an IL 12/23 therapeutic
b. Ozanimod is an oral S1P modulator
c. Ozanimod is an oral JAK inhibitor
4. A patient who visits the pharmacy often for medication related to his UC brings a few OTC products to the register. Which of the following might be a problem?
a. Simethicone
b. Naproxen
c. Curcumin
5. Which symptoms might a new patient suffering with UC reveal to a pharmacy technician?
a. cold sores, stomach ulcers, 1 BM/day, miss 1 day of work/year
b. bloody stools, 6 BMs/day, mood changes, had to quit work
c. 1 BM/day, cold sore, dental pain, perfect work attendance
6. You receive a prescription or order for tofacitinib. Which of the following would be an appropriate dose for a patient who has UC?
a. 5 mg orally once a week
b. 5-10 mg orally daily
c. 10 mg IV every 8 weeks
7. Which statement contains safety information to be aware of with UC treatments?
a. Sulfasalazine carries a risk for venous thromboembolism, herpes zoster, and major cardiovascular adverse events
b. JAK inhibitors may interfere with folate metabolism, affect male fertility, cause rare cutaneous adverse effects
c. Ustekinumab may cause infections, infusion or injection site reactions and may increase the risk of malignancy
8. A 22-year-old college student with newly diagnosed UC is experiencing daily blood in the stool, cramping, and had bowel incontinence on the way to the parking lot after class. Multiple options are available to treat this patient. What are the goals of treatment?
a. To prescribe medication to obtain response in 1 week and remission in 8 days
b. To induce and maintain clinical and endoscopic remission and quality of life
c. To use oral medications only (and avoid infusions) because of her youth and lifestyle
9. A 17-year-old restaurant worker presents with pancolitis. She has no other significant past medical history. She was treated with adalimumab, but the condition is worse with daily blood in the stool, 5 BMs/day, and weight loss of 10 pounds in the past 2 months. She has been reading about UC and is interested in switching to an infusion. Which is a possible IV treatment option?
a. Prednisone
b. Vedolizumab
c. Balsalazide
10. A 30-year-old newly married patient with UC works as a flight attendant. She has pancolitis and was told she is anemic and today has a fever. She comes to the pharmacy before her doctor’s appointment asking which UC medications are oral. Which product names are options to provide this patient?
a. Methylprednisolone
b. Ustekinumab
c. Ozanimod
References
Full List of References
REFERENCES
- Yu YR, Rodriguez JR. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin Pediatr Surg. 2017;26(6):349-355. doi: 10.1053/j.sempedsurg.2017.10.003
- Guindi M, Riddell RH. Indeterminate colitis. J Clin Pathol. 2004;57(12):1233-1244. doi: 10.1136/jcp.2003.015214
- Joo M, Odze RD. Rectal sparing and skip lesions in ulcerative colitis: a comparative study of endoscopic and histologic findings in patients who underwent proctocolectomy. Am J Surg Pathol. 2010;34(5):689-696. doi: 10.1097/PAS.0b013e3181db84cd
- Maaser C, Sturm A, Vavricka SR, et al. European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR] ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144-164. doi:10.1093/ecco-jcc/jjy113
- Kobayashi T, Siegmund B, Le Berre C et al. Ulcerative colitis. Nat Rev Dis Prim. 2020;6:74. doi: 10.1038/s41572-020-0205-x
- Segal JP, LeBlanc JF, Hart AL. Ulcerative colitis: an update. Clin Med (Lond). 2021;21(2):135-139. doi: 10.7861/clinmed.2021-0080
- Alatab S, Sepanlou SG, Ikuta K, et al.; GBD 2017 Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2020;5:17–30. doi: https://doi.org/10.1016/S2468-1253(19)30333-4
- Pilon D, Zhijie D, Muser E, et al. Long-term direct and indirect costs of ulcerative colitis in a privately-insured United States population. CurrMed Res Opin. 2020;36(8):1285-129. doi: 10.1080/03007995.2020.1771293
- Sturm A, Maaser C, Calabrese E, et al. European Crohn’s and Colitis Organisation [ECCO] and the European Society of Gastrointestinal and Abdominal Radiology [ESGAR] ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 2: IBD scores and general principles and technical aspects. J Crohns Colitis. 2019;13(3):273-284. doi: 10.1093/ecco-jcc/jjy114
- Trivedi PJ, Adams DH. Chemokines and Chemokine Receptors as Therapeutic Targets in Inflammatory Bowel Disease; Pitfalls and Promise. J Crohns Colitis. 2018;12(suppl_2):S641-S652. doi: 10.1093/ecco-jcc/jjx145
- Taleban S, Colombel JF, Mohler MJ, Fain MJ. Inflammatory bowel disease and the elderly: a review. J Crohn’s Colitis. 2015;9:507–15. doi: https://doi.org/10.1093/ecco-jcc/jjv059
- Xu F, Dahlhamer JM, Zammitti EP, Wheaton AG, Croft JB. Health-risk behaviors and chronic conditions among adults with inflammatory bowel disease—United States, 2015 and 2016. MMWR Morb Mortal Wkly Rep. 2018;67:190-5. doi: https://doi.org/10.15585/mmwr.mm6706a4
- Gajendran M, Loganathan P, Jimenez G, et al. A comprehensive review and update on ulcerative colitis. Dis Mon. 2019;65(12):100851. doi: 10.1016/j.disamonth.2019.02.004)
- Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389(10080):1756-1770. doi: 10.1016/S0140-6736(16)32126-2
- Lamb CA, Kennedy NA, Raine T et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68:s1–s106. doi: 10.1136/gutjnl-2019-318484
- Shah SC, Itzkowitz SH. Reappraising risk factors for inflammatory bowel disease-associated neoplasia: implications for colonoscopic surveillance in IBD. J Crohn’s Colitis. 2020;14:1172-1177. doi: 10.1093/ecco-jcc/jjaa040
- Terry R, Chintanaboina J, Patel D, et al. Expression of WIF-1 in inflammatory bowel disease. Histol Histopathol. 2019;34(2):149-157
- Yamamoto-Furusho JK, Fonseca-Camarillo G, Furuzawa-Carballeda J, et al. Caspase recruitment domain (CARD) family (CARD9, CARD10, CARD11, CARD14 and CARD15) are increased during active inflammation in patients with inflammatory bowel disease. J Inflamm (Lond).2018;15:13. doi: 10.1186/s12950-018-0189-4.
- Lewis JD, Chuai S, Nessel L, et al. Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis2008;14:1660–1666. doi:10.1002/ibd.20520
- Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317:1625–1629. doi: 10.1056/NEJM198712243172603
- Kornbluth A, Sachar DB, Practice Parameters Committee of the American College of Gastroenterology. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501-523; doi: 10.1038/ajg.2009.727
- Dipentum. Prescribing information. Viatris Inc.; June 2021. Accessed April 12, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/019715s029lbl.pdf
- Blackwell J, Saxena S, Jayasooriya N, et al. Prevalence and Duration of Gastrointestinal Symptoms Before Diagnosis of Inflammatory Bowel Disease and Predictors of Timely Specialist Review: A Population-Based Study. J Crohns Colitis. 2021;15(2): 203-211. doi:https://doi.org/10.1093/ecco-jcc/jjaa146
- Colman RJ, Dhaliwal J, Rosen MJ. Predicting Therapeutic Response in Pediatric Ulcerative Colitis-A Journey Towards Precision Medicine. Front Pediatr. 2021;9:634739. doi: 10.3389/fped.2021
- Finkelstein A. You Will Have a New Life. Ann Fam Med. 2018;16(2):166-167. doi: 10.1370/afm.2181
- Levine A, Griffiths A, Markowitz J, Wilson DC, Turner D, Russell RK, et al. Pediatric modification of the Montreal classification for inflammatory bowel disease: the Paris classification. Inflamm Bowel Dis.2011;17:1314–1321. doi:10.1002/ibd.21493
- Aloi M, Lionetti P, Barabino A, et al. Phenotype and Disease Course of Early-onset Pediatric Inflammatory Bowel Disease. Inflamm Bowel Dis. 2014;20(4):597-605.
- Levine A, de Bie CI, Turner D, et al. Atypical disease phenotypes in pediatric ulcerative colitis: 5-year analyses of the EUROKIDS Registry. Inflamm Bowel Dis.2013;19:370-377. doi:10.1002/ibd.23013
- Armuzzi A, Liguori G. Quality of life in patients with moderate to severe ulcerative colitis and the impact of treatment: A narrative review. Dig Liver Dis. 2021;53(7):803-808. doi: 10.1016/j.dld.2021.03.002
- Wang Y, Makadia R, Knoll C, Hardin J, Voss EA, Fife D, Davis K, Sloan S. Understanding patient journey in ulcerative colitis prior to biologic initiation: a 5-year exploration. BMC Gastroenterol. 2021;21(1):121. doi: 10.1186/s12876-021-01708-6.
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD. ACG clinical guideline: ulcerative colitis in adults. J Am Coll Gastroenterol.2019;114:384–413. doi: 10.14309/ajg.0000000000000152
- Peyrin-Biroulet L, Sandborn W, Sands B, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining Therapeutic Goals for Treat-to-Target. Am J Gastroenterol2015;110:1324-1338. doi: 10.1038/ajg.2015.233
- Colombel JF, D'haens G, Lee WJ, Petersson J, Panaccione R. Outcomes and Strategies to Support a Treat-to-target Approach in Inflammatory Bowel Disease: A Systematic Review. J Crohns Colitis. 2020;14(2):254-266. doi:10.1093/ecco-jcc/jjz131
- Ko C, Singh S, Feuerstein J, et al. American Gastroenterological Institute Guideline on the management of mild-moderate ulcerative colitis. Gastroenterology. 2019;156(3):748-764. doi: 10.1053/j.gastro.2018.12.009
- Diaz G. Mild-to-moderate ulcerative colitis. GrepMed. Updated July 31,2020. Accessed April 9, 2023. https://www.grepmed.com/images/9608/moderate-algorithm-management-colitis-treatment-
- Singh S, Feuerstein JD, Binion DG, Tremaine WJ. AGA Technical Review on the Management of Mild-to-Moderate Ulcerative Colitis. Gastroenterology. 2019;156(3):769-808.e29. doi: 10.1053/j.gastro.2018.12.008
- Feuerstein JD, Cheifetz AS. Ulcerative colitis: epidemiology, diagnosis, and management. Mayo Clin Proc. 2014;89(11):1553-63. doi: 10.1016/j.mayocp.2014.07.002
- Burri E, Maillard M, H, Schoepfer A, M, et al. Treatment Algorithm for Mild and Moderate-to-Severe Ulcerative Colitis: An Update. Digestion. 2020;101(suppl 1):2-15. doi: 10.1159/000504092
- Rinvoq. Prescribing information. Abbvie Inc; April 2023. Accessed April 11, 2023. https://www.rxabbvie.com/pdf/rinvoq_pi.pdf
- Xeljanz. Prescribing information. Pfizer Labs; January 2022. Accessed April 11, 2023. https://labeling.pfizer.com/ShowLabeling.aspx?id=959
- Stelara. Prescribing information. Janssen; July 2022. Accessed April 10, 2023. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/STELARA-pi.pdf
- Zeposia. Prescribing information. Bristol-Myers Squibb; November 2022. Accessed April 10, 2023. https://packageinserts.bms.com/pi/pi_zeposia.pdf
- Azulfidine. Prescribing information. Pfizer Labs: October 2022. Accessed April 11, 2023. https://labeling.pfizer.com/ShowLabeling.aspx?id=524
- Feuerstein JD, Isaacs K, Schneider Y, et al. American Gastroenterological Association Institute clinical guideline on the management of moderate to severe ulcerative colitis. Gastroenterology. 2020;158(5):1450-1461. doi: 10.1053/j.gastro.2020.01.006
- Rubin WA Jr, Loftus EV Jr, Harmsen WS, Zinsmeister AR, Sandborn WJ. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121(2):255-260. doi: 10.1053/gast.2001.26279
- Zyloprim. Prescribing information. Casper Pharma. December 2018. Accessed April 9, 2023 https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/016084s044lbl.pdf
- Strik AS, van den Brink GR, Ponsioen C, Mathot R, Löwenberg M, D'Haens GR. Suppression of anti-drug antibodies to inFLIXimab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45(8):1128-1134. doi:10.1111/apt.13994
- Kim M, Kim E, Kang B, Choe Y. Infliximab Therapy for Children with Moderate to Severe Ulcerative Colitis: A Step-Up versus a Top-Down Strategy. Yonsei Med J. 2021;62(7):608-614. doi: 10.3349/ymj.2021.62.7.608
-
Entyvio. Prescribing information. Takeda Pharmaceuticals USA, Inc.; June 2022. Accessed April 10, 2023. https://content.takeda.com/?contenttype=PI&product=ENTY&languag e=ENG&country=USA&documentnumber=1
- Sandborn WJ, Vermeire S, Peyrin-Biroulet L, et al. Etrasimod as induction and maintenance therapy for ulcerative colitis (ELEVATE): two randomised, double-blind, placebo-controlled, phase 3 studies. Lancet. 2023;401(10383):1159-1171. doi: 10.1016/S0140-6736(23)00061-2
- Rizankizumab (SKYRIZI®) achieves primary and all secondary endpoints in phase 3 induction study in patients with ulcerative colitis. Abbvie. News Center. March 23, 2023. Accessed April 10, 2023. https://news.abbvie.com/news/press-releases/risankizumab-skyrizi-achieves-primary-and-all-secondary-endpoints-in-phase-3-induction-study-in-patients-with-ulcerative-colitis.htm
- Remicade. Prescribing information. Janssen Biotech, Inc. October 2021. Accessed April 8, 2023
- Living with Ulcerative Colitis. Crohn’s and Colitis Foundation. December 2018. Accessed April 11, 2023. https://issuu.com/ccfa1/docs/living-with-ulcerative-colitis-brochure-final?fr=sN2ZhYjM3MDAxNzI
- Patrick E. Over the counter medication for ulcerative colitis. Ulcer Talk. April 10, 2023. Accessed April 15, 2023https://www.ulcertalk.com/over-the-counter-medication-for-ulcerative-colitis/