Learning Objectives
After completing this application-based continuing education activity, pharmacists will be able to
| 1. List the classifications of chronic kidney disease |
| 2. Describe the unmet medical needs, clinical practice guidelines, and scientific evidence in renal disease for patients with T2DM |
| 3. Review emerging information about COVID-19’s impact and optimal management of patients with chronic kidney disease |
| 4. Identify medications that are nephrotoxic and treatments that slow or prevent CKD |
| 5. Apply patient counseling skills to improve awareness of condition and adherence to treatment |
After completing this application-based continuing education activity, pharmacy technicians will be able to
| 1. List the basic pathology and symptoms of renal disease and diabetes |
| 2. Recall treatments used in patients who have comorbid renal disease and diabetes |
| 3. Identify when to refer patients to the pharmacists for recommendations or referrals |

Release Date:
Release Date: March 15, 2022
Expiration Date: March 15, 2025
Course Fee
FREE
ACPE UANs
Pharmacist: 0009-0000-22-021-H01-P
Pharmacy Technician: 0009-0000-22-021-H01-T
Session Codes
Pharmacist: 22YC21-JKT88
Pharmacy Technician: 22YC21-TKJ93
Accreditation Hours
2.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-22-021-H01-P/T will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Ashley Walsh, PharmD
Staff Pharmacist
Mohegan Pharmacy
Uncasville, CT
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Dr. Walsh has no relationship with ineligible companies and therefore has nothing to disclose.
ABSTRACT
Kidney disease’s main risk factor is diabetes. Approximately one in three patients with diabetes have chronic kidney disease (CKD). Hypertension and smoking also increase risk and speed of its progression. Healthcare providers have primarily directed prevention at strict blood pressure control, use of renin-angiotensin-aldosterone-system blockers, adequate glycemic control, and cardiovascular risk factor control. Patients rarely exhibit symptoms until CKD is advanced, making diagnosis and treatment a challenge. Early screening for renal disease prompts earlier intervention and delays progression. Pharmacists can apply guideline recommendations that intersect with their scope of practice and recognize the advantage of renal protective medications. Seemingly harmless over the counter medications, such as non-steroidal anti-inflammatory drugs (NSAIDs), can cause acute kidney injury. Technicians can take advantage of frequent patient interactions at the point of sale, screen for inappropriate NSAID use, and refer patients for pharmacist consultation. Pharmacy teams also need to be aware of coronavirus’s implications for patients with diabetes and offer support when appropriate.
CONTENT
Content
Introduction
Type 2 diabetes mellitus (T2DM) is a progressive metabolic disorder resulting from insulin resistance requiring lifelong management.1 Managing comorbidities involves a multifaceted approach to care. Diabetic kidney disease (DKD) is a leading cause of morbidity and mortality.2
This home-study will use both terms, DKD and chronic kidney disease (CKD). The Kidney Disease: Improving Global Outcomes (KDIGO) guideline avoids using DKD because it implies all kidney disease is caused by diabetes pathophysiology. However, KDIGO also deems the term DKD appropriate when clinicians recognize the limitation of diabetes causing kidney disease.3
Epidemiology, Risk Factors, and Symptoms
Worldwide, more than 450 million people have diabetes. This number is expected to exceed 700 million by 2045.4 Forty percent of these patients develop DKD and may go on to develop kidney failure requiring renal replacement therapy (RRT).4 Of note, rap musician Freeway, comedian Tracy Morgan, and actress Lucy Davis all have diabetes—and kidney disease.5
Unfortunately, CKD may be present at diagnosis of T2DM.6 It can markedly increase cardiovascular risk and health care costs.6 Reducing DKD’s prevalence requires management efforts and support for patients.7
Demographic factors associated with development and progression of CKD among patients with T2DM include7,8
- African American race
- Hispanic ethnicities
- Male sex
- Older age
Clinical factors include7,8
- Acute kidney injury (AKI)
- Cardiovascular disease (CVD)
- Family history of CKD, diabetes
- Hyperlipidemia
- Hypertension
- Obesity
- Smoking
Each kidney has about 1 million nephrons. Humans’ large physiologic reserve make slow loss unnoticeable. Often patients are asymptomatic until more than three quarters of kidney function is lost (usually stage 4 or 5), leading them to refrain from taking measures to treat the disease.9,10 Eventually, CKD progresses to end stage renal disease (ESRD).11
Unfortunately, patients do not realize they have renal dysfunction until symptoms begin to present. Examples of symptoms include 12
- Bleeding
- Cold intolerance
- Fatigue
- Itching
- Loss of appetite
- Mental confusion
- Nausea and vomiting
- Peripheral neuropathies
- Shortness of breath
- Weakness
Pathogenesis
Located below the rib cage in the middle of the back, each kidney is about the size of a closed fist and shaped like a kidney bean (Figure 1).13 The kidneys’ regulatory and excretory functions maintain balance. Regulatory function controls the blood’s composition and volume. The kidneys also maintain stable sodium, potassium, and calcium concentrations, along with acid-base balance. The excretory function removes metabolic wastes (e.g., uremia) and produces urine.8 The tubule returns needed and useful substances to blood, and waste and extra water becomes urine.14
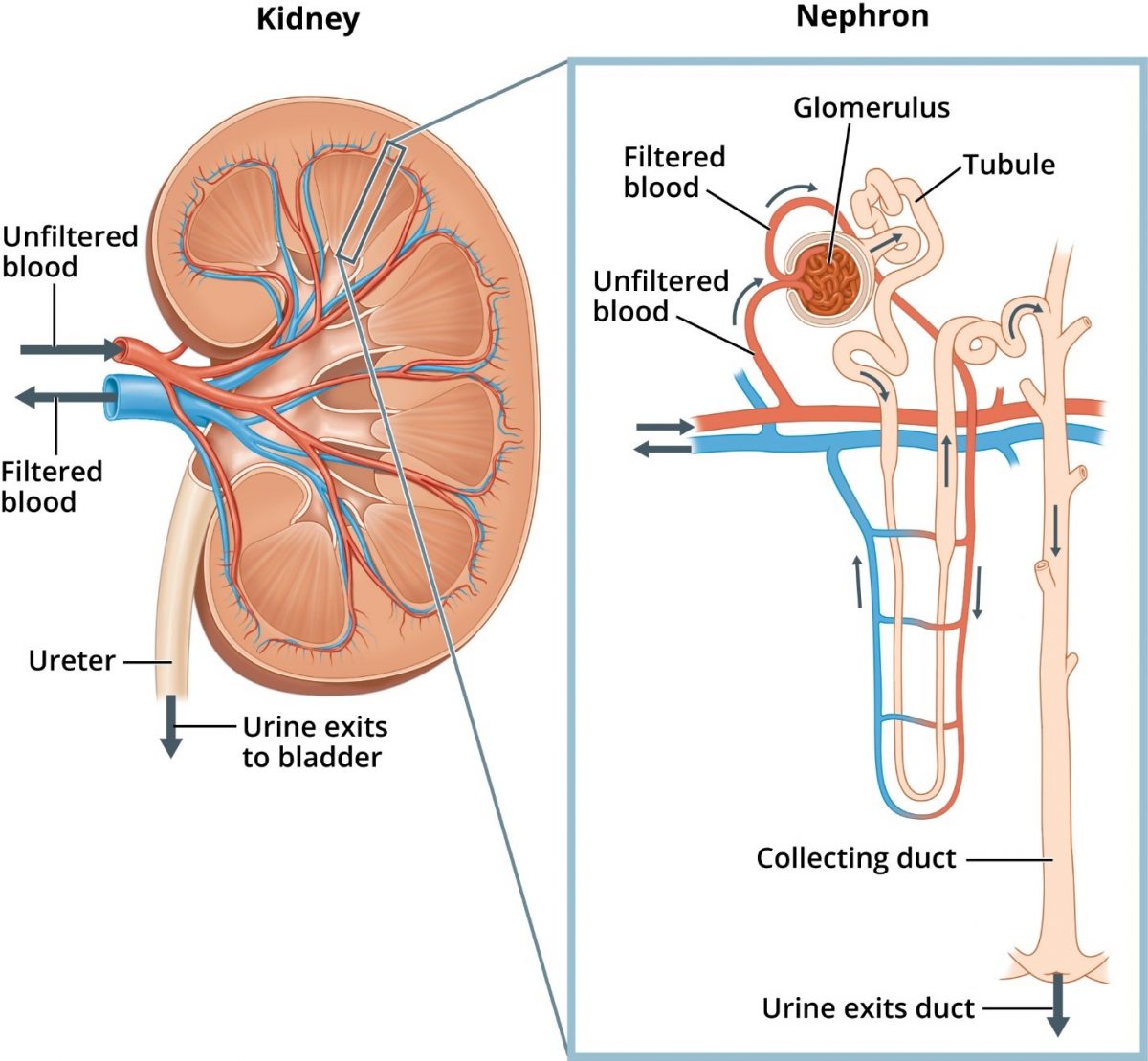
The kidneys have other functions. Their hormonal function produces renin for blood pressure (BP) control, generates erythropoietin that stimulates marrow production of red blood cells, and activates vitamin D needed for bone health. The kidneys also have metabolic functions contributing to glucose generation and metabolism of drugs and substances derived internally (e.g., insulin).8
DKD’s natural history starts with hyperglycemia. The kidneys begin to filter too much blood and the glomerular filtration rate (GFR) increases. The glomerulus acts as a filter and increased permeability allows albumin to cross the glomerulus into the urine. Higher albumin levels within the tubule may exacerbate kidney damage by exceeding the tubules’ ability to reabsorb.10 After years of working hard to filter more blood, the kidneys start to leak, and protein is lost in the urine (e.g., albuminuria).2,14 Albuminuria may be the first sign of DKD.8 Overtime, GFR slowly declines. Ultimately, the kidneys fail at ESRD.2
Increased pressure and flow within the glomerulus may damage nephrons and decrease nephron mass.8,12 The remaining nephrons then compensate using autoregulation. Renin release increases and converts angiotensin I to angiotensin II (ATII). ATII is a potent vasoconstrictor of afferent (e.g., brings blood to the glomerulus) and efferent (e.g., drains blood away from the glomerulus) arterioles – but preferentially affects efferent arterioles. This increases pressure within the glomerulus. More fluid reaches the kidneys and passes into the tubules. Consequently, tubular reabsorption increases.12
This compensatory mechanism may be adaptive and beneficial, but over time it can diminish the number of functioning nephrons. High pressure in the glomerulus impairs the size-selective function of the filter, resulting in more albumin in urine. Furthermore, ATII may mediate CKD progression through nonhemodynamic effects which ultimately results in further inflammation and fibrosis.12 Hypertension and obesity also contribute to high glomerular pressure and enlargement in T2DM.2 Metabolic changes, such as hyperglycemia, alter kidney hemodynamics and promote inflammation and fibrosis.2
Measuring Kidney Function
GFR is equal to the sum of the filtration rates in all functioning nephrons. An estimation of GFR (eGFR) uses the serum creatinine (SCr) level to measure the number of functioning nephrons roughly. eGFR does not actually measure the GFR. It estimates the measured GFR within +/- 85%. Small fluctuations in GFR are common and do not necessarily indicate disease progression.17 KDIGO guidelines consider progression at a minimal percentage change of at least 25%.17 Creatinine-based kidney function estimates have limitations. Results can be inaccurate with rapidly changing creatinine levels (e.g., in AKI), extremes in muscle mass or body size, or altered diet patterns, medications that interfere with SCr measurement, and use of creatine supplements.8 Medications associated with a false increase in SCr include antibiotics such as 9
- cefaclor
- cefazolin
- cefoxitin
- cephalexin
- clavulonic acid
Clinicians should measure SCr when antibiotic concentrations are at the lowest (at the end of dosing interval) to minimize this false increase.9 Additionally, medications can alter SCr through inhibition of active tubular secretion of creatinine. These include9
- amiodarone
- cimetidine
- cobicistat
- dolutegravir
- pyrimethamine
- rilpivirine
- trimethoprim
Approaches to estimate kidney function include Cockcroft-Gault, Modification of Diet in Renal Disease (MDRD), and Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI).10 Equations estimating renal function are less reliable if the patient has good kidney function. Estimates of kidney function are difficult to interpret in older patients because kidney function naturally declines with age.8 “Normal” serum creatinine levels listed on lab reports do not account for muscle mass, age, sex, and race.8
Each approach has limitations. Researchers developed the Cockcroft-Gault formula based on only 249 men aged 18 to 92.10 Women tend to have less muscle mass, thus, the equation assumes females would have a creatinine clearance 15% lower than men with the same SCr.10 MDRD and CKD-EPI are most widely used; the equations include adjustments for SCr, age, race, sex, and body surface area (see Table 1).10 KDIGO recommends using CKD-EPI for reporting eGFR in adults because the equation is more precise and accurate than MDRD, especially at GFR 60 mL/min/1.73m2 or greater.17
| Table 1. Comparison of Equations Used to Estimate Kidney FunctionH#,J#,O#,X1#,G2#,H2# | |||
| Name | Equation | Sex§ | Race§ |
| Cockcroft-Gault (mL/min) | = (140 – Age in years) * (IBW in kg)†
(72 * SCr in mg/dL)
|
0.85 if female
|
N/A |
| MDRD
(mL/min/1.73 m2) |
= 175 * (SCr in mg/dL)-1.154 * (Age in years)-0.203
|
0.742 if female | 1.212 if African American |
| CKD-EPI
(mL/min/1.73 m2) |
= 141 * min (SCr in mg/dL /κ, 1)α * max(SCr in mg/dL /κ, 1)-1.209 * 0.993Age in years
κ = 0.7 for females and 0.9 for males, α = -0.329 for females, -0.411 for males, min = minimum of Scr/κ or 1 max = maximum of Scr/κ or 1
|
1.018 if female | 1.159 if African American |
| §Multiplier; †Use ABW if ABW < IBW; use AdjBW when ABW > 130% IBW
ABW = actual body weight (in kg); AdjBW = adjusted body weight (in kg); IBW = ideal body weight; CKD-EPI = Chronic Kidney Disease Epidemiology Collaboration; MDRD = Modification of Diet in Renal Disease
|
|||
Unjustly, applying a racial multiplier delays recognition of CKD. The multiplier can falsely increase eGFR by as much as 21%, even in patients identical in every other way.18 Race is not a biologic characteristic, and application of a racial multiplier delays necessary treatments (e.g., dialysis or renal transplantation) and promotes inappropriate medication dosing.18,19 The National Kidney Foundation and the American Society of Nephrology Task Force now recommend the immediate removal of race as a variable in eGFR estimations because of its potential adverse consequences.19,20
Measuring kidney function is also vital when using medications that require renal dose adjustments. Clinicians should adjust doses of renally cleared drugs as described in their package inserts.21 The National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Laboratory Working Group recommends using eGFR (mL/min/1.73m2) or creatinine clearance (mL/min) for drug dosing.22 The NIDDK suggests performing a dosing conversion equation for very large or very small patients.slow22 This equation converts eGFR to units of mL/min.22
eGFR (mL/min) for drug dosing = eGFR (mL/min/1.73m2) x BSA/1.73
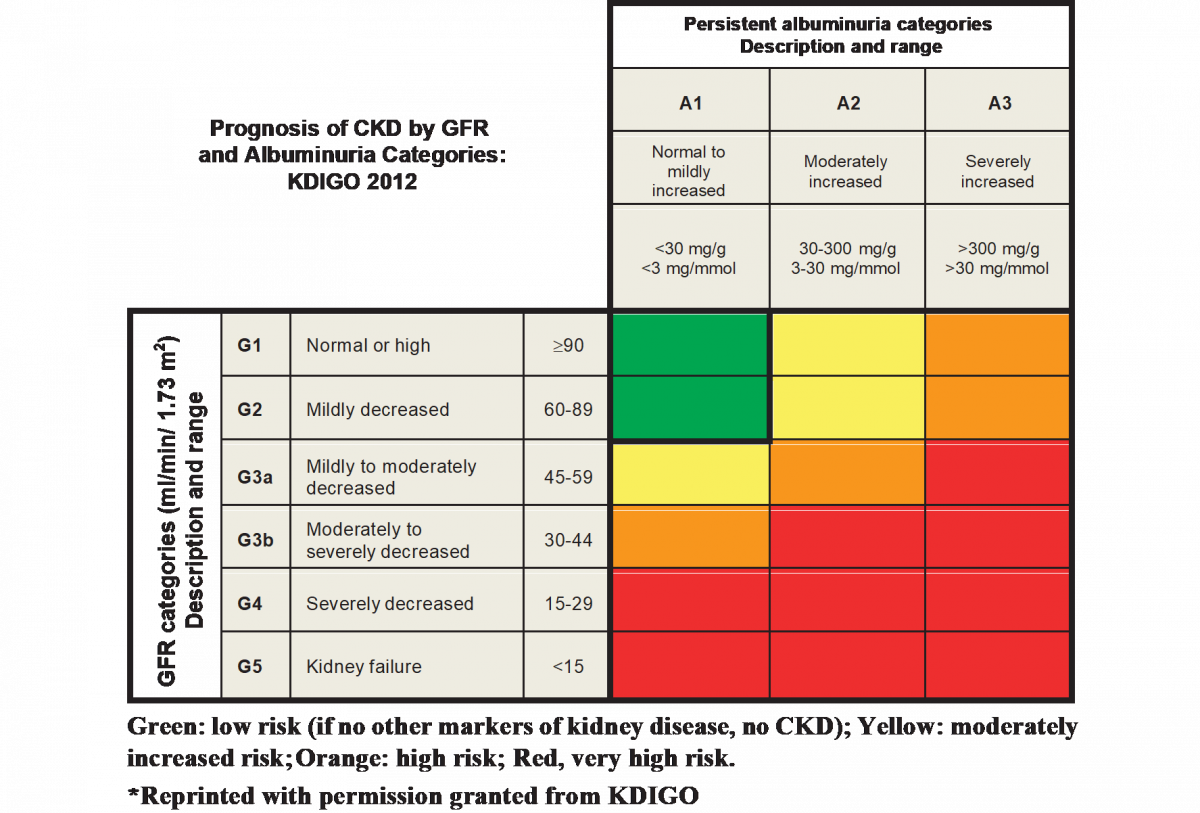
The KDIGO guidelines define CKD as abnormalities of kidney structure or function, present for more than three months, with implications for health.17 KDIGO classifies CKD (see Figure 2) based on cause, GFR category (G1-G4), and albuminuria category (A1-A3).23 A patient lacking evidence of kidney damage could not be classified as having CKD.24 Markers of kidney damage include 23
- albuminuria (albumin excretion rate greater than or equal to 30 mg/24 hours)
- electrolyte and other abnormalities due to tubular disorders
- histologic abnormalities detected by histology
- history of kidney transplantation
- structural abnormalities detected by imaging
- urinary sediment abnormalities
Screening/Monitoring & Diagnosis
DKD’s typical presentation includes retinopathy (which is often the first microvascular complication from long-standing of diabetes), albuminuria without visualized blood in the urine, and gradually progressive eGFR decline.6
Healthcare providers diagnose DKD based on the presence of albuminuria and/or reduced eGFR in the absence of signs or symptoms of other primary causes of kidney damage.6 Urine albumin-to-creatinine ratio (UACR) is a test to estimate 24-hour albumin excretion. UACR is a continuous variable and can increase temporarily from heart failure, episodic hyperglycemia, exercise within 24 hours, fever, infection, menstruation, and serious hypertension.6 Normal UACR is less than 30 mg/g.8 eGFR less than 60 mL/min/1.73m2 is considered kidney disease, and less than 15 mL/min/1.73m2 is kidney failure.8
Clinicians should assess patients with T2DM at least annually for CKD. Depending on the disease severity and progression, assessing more than four times annually may be necessary. The American Diabetes Association (ADA) recommends screening for urine albumin with UACR (on a spot urine specimen) and eGFR at least annually.6 Providers need to monitor patients with diabetes and urinary albumin exceeding 300 mg/g creatinine and/or an estimated glomerular filtration rate 30 to 60 mL/min/1.73m2 twice annually to guide therapy.6 Diagnosis of kidney injury requires confirmation with a second test within a three-to-six-month period.6,8
Pharmacists should closely monitor patients with eGFR less than 60 mL/min/1.73m2 to verify appropriate medication dosing, minimize exposure to nephrotoxins (e.g., NSAIDs and iodinated contrast), and evaluate potential CKD complications.6 Complications become prevalent at stage 3 and become more common and severe as CKD progresses. Elevated BP, volume overload, electrolyte abnormalities, metabolic acidosis, anemia, and metabolic bone disease are possible complications.6 At every clinical contact, clinicians should assess BP and volume status if possible. The ADA guidelines encourage clinicians to evaluate laboratory results every six to 12 months for stage 3 CKD, and more frequently for progressive stages, or as indicated to reassess symptoms or changes in therapy.6
Nephrology Referral
Physicians should refer patients to a nephrologist to assist with diagnostic or therapeutic challenges related to CKD complications (e.g., resistant hypertension, anemia, abnormal mineral metabolism and bone disorders, electrolyte disturbances).6 Nephrologists can help manage AKI, rapidly increasing albuminuria or nephrotic syndrome, and rapidly decreasing eGFR.6,8 Diagnosis of advanced kidney disease (eGFR less than 30 mL/min/1.73m2) warrants a referral to discuss RRT for ESRD.6
At CKD stage 5, dialysis or kidney transplantation become essential for survival.25 The first human was dialyzed in 1924. Eventually, dialysis became more common and in addition to the growing number of patients initiating treatment, a significant number of patients required ongoing therapy. By the early 1960s, a finite number of nephrologists and facilities offering dialysis led to dialysis rationing (restricting the number of patients who could receive it).26 The federal government established an ESRD Medicare program in 1972 in response. Before that year, an anonymous committee selected patients for dialysis partly based on their social worth. The committee judged applicants based on their potential to remain, or become, productive community members. Determinants of social worth included level of education, marital status, net worth of patients, work performance and history, and number of dependents.27
Managing Renal Disease
Treatment’s goal is to delay or prevent progression of CKD and its associated complications.12 Early detection and early intervention minimizes adverse outcomes.11 Reaching blood glucose (BG) and BP goals are the best ways to slow and prevent DKD.28 When interpreting glucose parameters, clinicians should remember that hemoglobin A1C (HbA1C) values may be falsely low in patients with CKD. HbA1C is measured based on assumed red blood cell life span of 90 days. However, in patients with CKD, the red blood cell life span is shorter.12
Interventions for reducing urine albumin—controlling BP, reducing sodium intake, reducing weight if obese, reducing protein intake if excessive, and achieving tobacco cessation—may lower risk of progression.8
CVD Risk Reduction
To reduce cardiovascular risk, statins, beta blockers (BB), angiotensin converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB), and antiplatelet drugs are standard of care.12 The following section provides information on ACEi/ARB. Statins do not appear to slow CKD, but they may reduce urine protein and significantly reduce all-cause CVD mortality.29
Inhibition of RAAS
In 1934, Harry Goldblatt brought recognition to kidneys’ importance in BP control with his experiment in dogs. Goldblatt clamped the renal arteries, which precipitated chronic hypertension. This discovery led to investigation of the pressor substance. Two separate research groups in Argentina and America simultaneously identified the pressor substance that increases BP in response to renin. The pressor identified was later named “angiotensin.”30 Key trials helped scientists acknowledge that renin-angiotensin-aldosterone-system (RAAS) antagonists slow kidney disease progression independent of their effects on BP.4,31,32,33,34 The Ramipril Efficacy In Nephropathy trial demonstrated a renoprotective effect that exceeds the protection expected for the degree of BP lowering.31 Ramipril was well tolerated in advanced renal disease, continuing to prevent additional renal impairment.31 A study conducted in China found patients with SCr levels exceeding 3 mg/dL tolerate benazepril.34 The Irbesartan Diabetic Nephropathy Trial noted the mechanism responsible for renoprotection of ARB includes restriction of intrarenal angiotensin activity.32 The Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan study extrapolated observed data, estimating losartan delayed dialysis or transplantation by about two years.33
Antihypertensive medications such as ACEi and ARB block the RAAS and lower urine albumin. ACEi have names ending with “-pril” and may have a side effect of dry cough. ARB have names ending with “-sartan.” KDIGO guidelines suggest physicians titrate ACEi or ARB to the highest approved dose tolerated.4 The NIDDK recommends monitoring patients closely using ACEi or ARB for hyperkalemia and increases in SCr.10,12 KDIGO advises monitoring serum potassium and SCr levels within two to four weeks of initiation or when doses change.4 If hyperkalemia develops, clinicians may consider moderating patient potassium intake or introducing a loop diuretic to increase urinary potassium excretion.4,29 NIDKK emphasizes individualizing dietary potassium restriction for each patient to achieve and maintain a safe serum potassium (less than 5 mEq/L).29 Practice guidelines recommend continuing therapy despite minor increases in SCr (less than 30%).4 Guidelines encourage dose reductions or discontinuation for symptomatic hypotension, uncontrolled hyperkalemia, and AKI.4
| Table 2. Available SGLT-2i | ||
| Medication | Medication | Renal Considerations |
| canagliflozin | · 100 mg daily, titrated to 300 mg daily taken before first meal of the day
· Patients with eGFR 30-59 mL/min/1.73m2 should receive maximum 100 mg daily · Dose adjustment required when taken concomitantly with UGT inducers (rifampin, phenytoin, phenobarbital, ritonavir) |
· Initiation not recommended, and likely ineffective for glycemic control with eGFR
< 30 mL/min/1.73m2 · Contraindicated in dialysis |
| dapagliflozin | · 5 mg daily, titrated to 10 mg daily | · Likely ineffective for glycemic control with eGFR
< 45 mL/min/1.73m2 · Initiation not recommended with eGFR < 25 mL/min/1.73m2 · Contraindicated in dialysis |
| empagliflozin | · 10 mg daily, titrated to 25 mg daily as tolerated
· Taken in the morning with or without food |
· Do not use when eGFR < 30 mL/min/1.73m2
· Contraindicated in dialysis |
| ertugliflozin | · 5 mg daily, titrated to 15 mg daily as tolerated
· Taken in the morning with or without food |
· Initiation not recommended in patients with an eGFR 30-59 mL/min/1.73m2
· Continued use not recommended in patients with an eGFR consistently < 60 mL/min/1.73m2 · Contraindicated in eGFR < 30 mL/min/1.73m2, ESRD, or dialysis |
| eGFR = estimated glomerular filtration rate; ESRD = end stage renal disease; SGLT-2i = sodium glucose co-transporter 2 inhibitors; UGT = uridine 5’-diphosphate glucuronosyltransferase
|
||
Phlorizin, a natural product derived from the root bark of apple trees, provided the chemical starting point for modification and achievement of selective sodium-glucose transporter 2 inhibitors (SGLT-2i).35 Currently, four SGLT-2i are approved: canagliflozin, dapagliflozin, empagliflozin, and ertugliflozin (Table 2).39,40 SGLT-2i, along with metformin, are the recommended therapy for patients with T2DM, CKD, and eGFR greater than or equal to 30 mL/min/1.73m2.4 Trials have reported consistent reductions in cardiovascular events and CKD progression.4 SGLT-2i provide an HbA1C reduction of 0.5-1% and 1 t0 5 kg of weight loss from visceral fat (not muscle mass loss).39 They also reduce systolic BP by 3 to 4 mmHg and diastolic BP by 1 to 2 mmHg.39
The Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial showed canagliflozin, in addition to RAAS blockade, preserves renal function and improves cardiovascular outcomes.41,42 SGLT-2i benefits appear independent of their BG decrease.41 Their effects may be mediated by natriuresis and glucose-induced osmotic diuresis, reducing intraglomerular pressure.42 The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial was designed to assess dapagliflozin’s long-term efficacy and safety.42 Researchers found a sustained decline in eGFR.42 In contrast to the CREDENCE trial, the DAPA-CKD trial included patients without T2DM and an eGFR below 30 mL/min/1.73m2.42 Patients treated with SGLT-2i, as expected, experience an initial dip in eGFR, followed by a stabilization of kidney-function decline.42
SGLT-2i reduce tubular glucose reabsorption.6,39 An insulin-independent mechanism lowers BG.39 As renal impairment continues, the efficacy of SGLT-2i decreases because the amount of glucose that reaches the proximal tubules declines as GFR declines.39
SGLT-2i are second line therapy in the ADA algorithm, and third line in the American Association of Clinical Endocrinology/American College of Endocrinology (AACE/ACE), guideline.6,39 Older adults with stage 4 or 5 CKD are poor candidates because of their typically diminished renal function and poor thirst response when dehydrated.39
Pharmacists have an opportunity to educate patients starting SGLT-2i about the secondary effects of modest volume contraction, BP reduction, and weight loss.4 Patients who are on diuretics are already at risk for hypovolemia.4 Lowering the diuretic dose and monitoring for orthostatic hypotension is recommended.4,39
The most common adverse effect is genitourinary infection, which is not dose dependent.39 Another side effect, symptomatic hypotension, occurs more frequently in patients with eGFR less than 60 mL/min/1.73m2.39
Post-marketing research has linked SGLT-2i and euglycemic diabetic ketoacidosis in case reports.39 Risk factors are dehydration, long standing T2DM, and serious illness.39 Ensuring patients are well hydrated before starting therapy and temporarily stopping if serious illness occurs is important.39 Patients using insulin should not decrease the dose when initiating SGLT-2i.39 Hypoglycemia is uncommon with SGLT-2i unless used in combination with sulfonylureas, meglitinides, or insulin.39
GLP-1RA
Glucagon-like peptide-1 receptor agonists (GLP-1RA) have positive effects on cardiovascular and renal events in DKD.43 They also offer potential adequate glycemic control in multiple stages of DKD, without increased hypoglycemic risk.43 GLP-1RA prevent macroalbuminuria’s onset and slows GFR decline.43 Additional benefits include weight reduction (1 to 2 kg) and lower HbA1C 0.5 to 1.5% when used as monotherapy.1,39,43
GLP-1RA increase insulin secretion in response to nutrients, particularly glucose (incretin effect), and suppress inappropriately high postprandial glucagon secretion from the pancreas. Reduced postprandial glucose levels follow.43 Weight loss results from increased satiety signals, slowed gastric emptying, and appetite reduction.39
The incretin effect is an increased insulin secretion in response to an oral glucose stimulus that promotes the pancreas to secrete insulin. Patients with T2DM have an incretin effect approximately half of that seen in nondiabetic patients.39
Hypoglycemia is an uncommon side effect because GLP-1RA enhance insulin secretion in a glucose dependent manner. Low BG is more common when combined with a sulfonylurea or insulin.1
GLP-1RA have beneficial direct and indirect effects. Reducing hyperfiltration, inflammation, oxidative stress, glomerular atherosclerosis, and ATII and renin concentrations are the direct effects.43 Improved glycemic control, increased insulin sensitivity, decreased plasma insulin levels, weight loss, and improved BP (1-2 mmHg) are the indirect effects.43
KDIGO recommends long-acting GLP-1RA for patients not reaching glycemic targets or those unable to use SGLT-2i.4 The AACE/ACE and ADA guidelines recommend GLP-1RA as second line therapy.39
Researchers took almost 20 years from identification of endogenous glucagon-like-peptide-1 to the first therapeutic GLP-1RA approval. Surprisingly, the first GLP-1RA approved was a peptide originally isolated from lizard venom (exenatide).44 GLP-1RA consists of two groups: incretin mimetics (exedin-4 analogs) and human GLP-1RA.43 Daily exenatide, weekly exenatide, and lixisenatide are approved exedin-4 analogs.43 Liraglutide, semaglutide (oral and subcutaneous), and dulaglutide are approved human GLP-1RA.43
Human GLP-1RA and weekly exenatide are long acting. The longer half-life contributes to suppressing glucagon overnight and improves fasting glucose.39 Patients taking long acting GLP-1RA experience less nausea due to a lower impact on gastric emptying.39 Nausea upon initiation appears to be dose-related and prescribers should titrate doses (Table 3).39 Gastrointestinal adverse effects decrease over time.39 Longer acting GLP-1RA are contraindicated in patients with a history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2 (higher risk of C-cell tumors).39
| Table 3. Available GLP-1 RA D1#,F1#,G1#,H1#,I1#,J1#,K1#,L1# | ||
| Medication | Dosing and Administration | Renal
Considerations |
| dulaglutide | · 0.75 mg SC once weekly; increase to 1.5 mg once weekly
· Maximum 4.5 mg after at least 4 weeks on previous dose · Administer with or without food · Administer a missed dose as soon as possible if at least 3 days (72 hours) until next scheduled dose |
None |
| exenatide | · 5 mcg SC twice daily and titrate to 10mcg twice daily in 1 month
· Inject up to 60 minutes before morning and evening meals. If patient does not eat breakfast, administer first injection of the day at lunch (at least 6 hours between doses). · Skip missed dose and resume with the next scheduled dose |
Avoid in patients with CrCl < 30 mL/min; caution when initiating or escalating doses in patients with CrCl 30-50 mL/min |
| exenatide extended release | · 2 mg SC every 7 days without regards to meals
· Administer missed dose as soon as possible if there are at least 3 days (72 hours) until the next scheduled dose |
Not recommended if eGRF < 45 mL/min/1.73m2 |
| liraglutide
|
· 0.6 mg SC daily (nontherapeutic to minimize side effects), then increase to 1.2 mg daily
· Maximum dose 1.8 mg/day · Skip missed dose and resume with next scheduled dose. If more than 3 days elapsed since last dose, reinitiate, and titrate per prescriber |
None |
| lixisenatide | · 10 mcg SC once daily for 14 days, then increase to 20 mcg daily
· Administer 1 hour before first meal · Missed dose administered 1 hour prior to next meal |
Avoid in patients with ESRD |
| semaglutide
(injectable) |
· 0.25 mg SC once weekly without regards to meals; increase to 0.5 mg after 4 weeks. May increase to 1 mg 4 weeks later
· Administer within 5 days of missed dose; if more than 5 days have passed, skip missed dose and administer on the next regularly scheduled day |
None
|
| semaglutide
(oral) |
· 3 mg PO daily for 30 days, then increase to 7 mg daily. Maximum 14 mg daily at least 30 days after 7 mg dose
· Take 30 minutes before first food, beverage, or other oral medications with no more than 4 oz plain water · Skip missed dose and resume taking the following day |
None |
| GLP-1 RA = glucagon-like peptide-1 receptor agonists
SC = subcutaneously ESRD = end stage renal disease CrCl = creatinine clearance eGFR = estimated glomerular filtration rate PO = by mouth |
||
Daily exenatide and lixisenatide are short acting and demonstrate a more pronounced decrease in postprandial glycemia.43 More nausea is associated with shorter acting GLP-1RA.39 Patients can limit nausea by eating slowly and stopping when full.39
Potentially, protein and peptide medications are immunogenic, meaning they can trigger an unwanted immune response against themselves. Thus, antibody formation may occur in patients taking GLP-1RA. The incidence of antibodies cannot be directly compared between products. Antibody formation has not been associated with reduced efficacy or increased side effects from GLP-1RA.39 The exception is extended release exenatide: patients may have an attenuated HbA1C response.51 Local injection site reactions are prominent in antibody positive patients. Prescribers can consider alternative therapy if patients experience worsening glycemic control or failure to achieve target glycemic control.39
Pause and Ponder: What would you tell a patient concerned about experiencing nausea upon starting GLP-1RA?
Glycemic Control
Hyperglycemia is T2DM’s hallmark feature. Glycemic control is fundamental to disease management.6 Glomeruli filter glucose which is then almost completely reabsorbed by proximal tubules.29 Glucose in the urine (glucosuria) occurs when the tubules’ ability to reabsorb glucose is exceeded. The renal threshold for glucose is 180-200 mg/dL.29
A lag time of at least two years exists between intensive glucose control and attenuated renal function decline.52 Intensive control early in diabetes offers a long-lasting, favorable effect on the risk of DKD development. Irreversible damage from hyperglycemia can be prevented.2
The AACE/ACE recommend targeting more stringent goals for glycemic control (HbA1C less than 6.5%) in younger patients with a shorter duration of diabetes, absence of complications, and longer life expectancy.53 The AACE/ACE encourages less stringent goals (H1A1C less than 8%) for older patients with longstanding diabetes, micro- and macrovascular complications, and limited life expectancy.2,29,53 KDIGO guidelines recommend preventing or delaying progression of diabetes’ microvascular complications by targeting HbA1C to about 7%. Providers should avoid treating patients at risk for hypoglycemia, such as those with DKD, to HbA1C less than 7%.2 The ADA agrees that tightfisted goals are inappropriate for patients at risk for hypoglycemia.6
Tight glucose control does not slow progression once patients have established DKD since circulating levels of insulin are higher due to reduced catabolism.29 Hypoglycemia risk increases as CKD advances: physicians should discontinue or adjust medications accordingly.29 For example, they should discontinue metformin at eGFR less than 30 mL/min/1.73m2.29 Renal function status does not restrict insulin doses, allowing titration upwards to reach glycemic goals.7 Unexplained eGFR improvement or increased hypoglycemia may indicate CKD is progressing.29
BP Control
Controlling BP is key to slowing progression of CKD, breaking a potentially vicious cycle associating hypertension and CKD.17 The ADA endorse a target BP less than 140/90 mmHg.6 A baseline systolic BP greater than 140 mmHg in patients with T2DM has been associated with higher risk of ESRD and death.2 The American College of Cardiology/American Heart Association (ACC/AHA) 2017 guidelines recommend a BP goal of 130/80 mmHg for all patients.54 Uncontrolled hypertension (systolic BP greater than 160 mmHg) is a major challenge and becomes an issue when elevated albuminuria goes unresolved.29
The ACC/AHA recommends introducing medication at a systolic BP greater than or equal to 130 mmHg or diastolic BP greater than or equal to 80 mmHg.54 Patients often require multiple medications to control BP.29,54 Drugs that reduce cardiovascular events include ACEi, ARB, thiazide-like diuretics, and calcium channel blockers (CCB).43 Combination of ACEi with ARB is harmful and should be avoided.4 The ACC/AHA advises initial antihypertensive treatment with a thiazide diuretic, CCB, and ACEi or ARB.54 In the Black population, ACC/AHA recommend initial treatment with a thiazide diuretic (especially chlorthalidone) or CCB.54 Physicians should assess patients one month after starting therapy to optimize medication adherence and consider treatment intensification.54 Physicians can reassess patients who have met their BP goal in three to six months.4,54
PRO TIP: Ask patients if they are interested in having their blood pressure checked at the pharmacy.
Diet
Weight loss induces a significant and rapid reduction in urine protein.43
Limiting salt and sodium is the first step to improve diet and enhances RAAS antagonists’ effects on lowering urine albumin.28,29 Even small reductions in sodium intake lowers BP.29 Less than 2300 mg of sodium each day can help control BP.14 For reference, about one teaspoon of salt has roughly 2300 mg of sodium.29
How can patients achieve such a seemingly difficult goal? 29
- Buy fresh. Prepared and packaged foods usually have added sodium.
- Cook food at home instead of eating frozen dinners or restaurant prepared food – control what goes into meals.
- Use spices, herbs, and sodium-free seasonings to add flavor instead of salt.
- Minimize salt by rinsing canned foods with water.
Heart-healthy foods (lean meats, fish, beans, vegetables, low-fat milk/yogurt/cheese, poultry without skin) minimize fat accumulation in the blood vessels, heart, and kidneys.14,29 Individuals should strive to keep both saturated fats and added sugar at less than 10% of total calories. Patients can grill, broil, or roast instead of frying. Using nonstick cooking spray minimizes the fat from using butter.29 Alcohol consumption should be in moderation (up to one drink/day for women and two for men).29 One drink is 12 ounces beer, 5 ounces wine, or 1.5 ounces liquor.29
As kidney function declines, patients may need to eat food with less phosphorus and potassium.14 In CKD, phosphorus accumulates in blood and pulls calcium from bones.14 Bones become thin, weak, and more likely to break.14 High levels of phosphorus cause noticeable symptoms, namely itchy skin, and bone and joint pain.14 Healthcare providers may add a prescription phosphate binder with meals to help remove excess phosphorus.14
How can patients limit phosphorus and potassium intake?14
- Ask a butcher to help choose fresh meats without added phosphorus.
- Choose food lower in phosphorus: fresh fruits and vegetables, breads, pasta, rice, rice milk (not enriched), corn and rice cereals, light-colored sodas.
- Stay away from food higher in phosphorus: meat, poultry, fish; bran cereals and oatmeal, dairy foods, beans, lentils, nuts, dark-colored soda.
- Read the label – salt substitutes may be high in potassium.
- Choose foods lower in potassium: apples, peaches, carrots, green beans, white bread and pasta, white rice, rice milk (not enriched), cooked rice and wheat cereals, grits, apple/grape/cranberry juice.
- Avoid food higher in potassium: oranges, bananas, orange juice, potatoes, tomatoes, brown and wild rice, bran cereals, dairy foods, whole-wheat bread and pasta, beans, and nuts.
Patients may find it beneficial to visit a registered dietician for medical nutrition therapy.8 Registered dieticians can assist with HbA1C lowering.8
Uncommonly known, any juice treats hypoglycemia.29 If patients are taking ACEi or ARB, discuss using glucose tabs or low-potassium juice to treat low BG.29 Juices with lower potassium content than the typically recommended orange juice include grapefruit, pineapple, grape, apple, cherry, and cranberry cocktail (from most to least potassium).29
Exercise
Physical inactivity is associated with increased mortality in CKD.29 Researchers agree that although data is limited, recommending activity may be beneficial. Since the risk of hypoglycemia is amplified during and after exercise, counseling patients on how to best manage hypoglycemia symptoms is necessary.29
Avoiding Nephrotoxins and OTC Recommendations
All patients with CKD have an increased AKI risk.17
Frequently, patients take ACEi or ARB, a diuretic, and start a non-steroidal anti-inflammatory drug (NSAID). This is known as a “triple whammy.” 55 This combination causes a problem because the ACEi or ARB vasodilate the efferent arteriole and diuretics decrease blood volume, limiting perfusion.55 Adding an NSAID prevents vasodilation of the afferent arteriole, so very little perfusion within the glomerulus causes reduced glomerular pressure, reduced filtration, and AKI.55
NSAIDs are universally accessible and disrupt blood flow to the kidneys.55 Upon discontinuation of NSAIDs, SCr may decrease and eGFR may increase.29 Pharmacy technicians have an opportunity to identify patients purchasing over the counter (OTC) NSAIDs and refer them to the pharmacist for consultation. Pharmacists can identify patients with advanced kidney disease based on their medication profile. Typically, patients are taking ACEi or ARB, phosphate binders (calcium acetate, sevelamer carbonate, lanthanum carbonate), vitamin D analogues (calcitriol, paricalcitol, doxercalciferol), calcimimetics (cinacalet), and erythropoiesis stimulating agents (epoetin alfa, darbepoetin alfa).55
Patients seeking relief for their headaches, pain, fever, or colds may unknowingly be taking NSAIDs because products are sold under many different brand names. Technicians can monitor purchasing of AKI inducing products containing naproxen, ibuprofen, and aspirin.14 A technician PRO TIP: call the pharmacist over for patients purchasing OTC pain and cold medications. This quick and easy intervention can have a meaningful impact on clinical outcomes. It is an opportunity to counsel on self-care products and direct the patient to safer alternatives.55 Pharmacists can direct patients to cold medications without NSAIDs. The National Kidney Foundation supports using oral acetaminophen for fever or pain.56 Pharmacists can suggest patients try OTC topical analgesics such as lidocaine or capsaicin (available as cream or patches) for pain.57 Patients may wish to try nonpharmacologic interventions such as transcutaneous electrical stimulation, topical heat and cryotherapy (warm and cold compresses), massage, acupuncture, and exercise.57
Pause and Ponder: What OTC medications would you suggest for patients who cannot take NSAIDs?
Healthcare providers can remember additional nephrotoxic medications by the acronym CARNIVAL CAMP; calcineurin inhibitors, ACEi/ARB, radiocontrast, NSAIDs, ifosfamide, vancomycin, aminoglycosides, lithium, cisplatin, amphotericin B, methotrexate, and penicillins.58 Although ACEi and ARB can be nephrotoxic, they are proven to be renoprotective for patients with DKD and urine albumin excretion greater than 30 mg/24 hr.58 They lower albuminuria.58
Patients feeling unwell or dehydrated need medical advice to develop a sick plan. Dehydration, in combination with some medications, worsens kidney function.59 The sick plan temporarily discontinues drugs excreted by the kidneys.12,59 These include digoxin, diuretics, lithium, metformin, NSAIDs/COX II inhibitors, RAAS blockers, SGLT-2i, and sulfonylureas.12,59
Empowering Patients and Improving Adherence
CKD remains under diagnosed.10 The Centers for Disease Control and Prevention reports as many as 90% of adults with CKD do not know they have CKD.60 Patients report feeling “fine” and symptoms may not be obvious until disease is advanced.29 Self-management of diabetes and hypertension can slow kidney disease progression, making patient awareness important. Guidelines for CKD and national programs recommend patient education as a critical component of care.61 Pharmacist counseling empowers patients in self-management of their CKD risks, improves well-being, and optimizes quality of life.61,62 Patients with CKD must understand their condition to participate in care decisions and planning.61 Pharmacists can provide education to minimize long-term complications.
Pharmacists can also play a part in diagnosis by discussing risk with T2DM patients and communicating the importance of testing for CKD. Pharmacists can identify these patients by scanning their medication profile for metformin (first line medication).39 PRO TIP: Use the pharmacy’s technology, or even a sticky note, to flag metformin prescriptions that are ready for pick-up for patient counseling. Many patients have little to no knowledge of diagnosis or dialysis. This poses a challenge when initiating treatment to delay disease progression. Pharmacists can educate patients regarding complications (e.g., heart disease), CKD’s progressive nature, treatment basics, and terminology (e.g., GFR).10,29 Patients require repetition to integrate the information learned.63 Fortunately, pharmacists are accessible to provide the repeated emphasis patients desire.
Pause and Ponder: What other health conditions present minimal symptoms until they have advanced?
As CKD advances, prevalence of medication-related problems and significant costs to the healthcare system increase because medication use increases. Clinicians prescribe six to eight medications, on average, for patients with stage 3 and 4 CKD. Patients with stage 5 are prescribed about 12 medications to treat five or six chronic medical conditions.10 Pharmacists can identify and resolve drug therapy problems between visits with providers.2 Failure to do so could accelerate disease progression.64 Pharmacists can monitor medication profiles for interactions and adjust doses based on kidney function.12 Additionally, pharmacists and patients need to discuss hypoglycemia risk, cost, and side effects.65 Communication ensures treatment satisfaction when patients are weighing their options and buying into adherence of their chosen regimen to help manage CKD.64,66
COVID-19 and T2DM
The relationship between coronavirus disease 2019 (COVID-19) and diabetes has been described as “the interaction of two pandemics.”67 Diabetes is the most common non-communicable chronic disease globally, and, one of the major comorbidities in patients with COVID-19.67 As many as 40% of the COVID fatalities–120,000 Americans–have been people with diabetes.68 Even if patients are not affected by infection, they still face challenges introduced by the virus. A new nationwide survey conducted by the ADA in partnership with Thrivable and the Diabetes Daily community uncovered the crisis Americans with diabetes are facing during the pandemic. The results of the survey acknowledge COVID-19 has created dangerous hurdles to accessing health care such as 68
- delaying routine medical care because patients fear exposure to COVID-19
- financial constraints limiting technology and insulin supplies
- disruptions in health insurance resulting from lost jobs
- reduced food access
Living with T2DM is a risk factor associated with developing more severe COVID-19 and increased risk of death.69 It is also associated with neurological complications.70 Furthermore, these patients are at increased risk for multi-organ dysfunction. Amid the burden on healthcare systems, clinicians must recognize these risks and focus on individualized treatment to avoid poor prognosis.69
Experts mostly consider COVID-19 a respiratory illness, but the kidneys may be one of its targets. The virus enters cells through angiotensin-converting enzyme 2 receptors, found abundantly in the kidney. Information on kidney involvement in COVID-19 is limited but evolving rapidly.71 COVID-19 directly attacks pancreatic islets and deteriorates glycemic control in patients with diabetes.67 Tight glucose monitoring and careful considerations of potential drug interactions are paramount to optimize outcomes for critically ill and hospitalized patients.67 Insulin is the safest choice for antihyperglycemic treatment because of the uncertainty of using some oral medications with COVID-19 infection.69
Continuing ACEi, statins, and oral or inhaled and intranasal corticosteroids prescribed for comorbid conditions after diagnosis of COVID-19 is crucial.70
Conclusion
Pharmacy teams can benefit from advanced knowledge to manage patients with DKD. The more routinely healthcare professionals screen patients for renal disease, the earlier progression can be delayed. Pharmacists can recommend renoprotective medications such as ACEI, ARB, SGLT-2i, and GLP-1RA to patients when appropriate. Technicians can take advantage of frequent patient interactions and advise them to consult with the pharmacist. Screening at the point of sale can discourage use of nephrotoxic OTC medications. Recurrent patient engagement translates into self-awareness, allowing for earlier intervention. Ultimately, clinicians can enhance patient care with better adherence, satisfaction of treatment, and improved quality of life.
Pharmacist Post Test (for viewing only)
Pharmacist Post-test
After completing this continuing education activity, pharmacists will be able to
• Describe the unmet medical needs, clinical practice guidelines, and scientific evidence in renal disease for patients with T2DM.
• List the classifications of chronic kidney disease.
• Review emerging information about COVID-19’s impact and optimal management of patients with chronic kidney disease.
• Identify medications that are nephrotoxic and treatments that slow or prevent CKD.
• Apply patient counseling skills to improve awareness of condition and adherence to treatment.
1. Which medication is nephrotoxic?
A. vancomycin
B. levofloxacin
C. azithromycin
2. Which eGFR category is associated with kidney failure?
A. G1
B. G5
C. G0
3. What is the SAFEST choice for antihyperglycemic treatment in T2DM patients with COVID-19?
A. insulin
B. glipizide
C. sitagliptin
4. A patient tells you he does NOT want to start a GLP1-RA because he is worried about experiencing nausea. What can you recommend?
A. Eat slowly and stop when full
B. Use a shorter acting GLP1-RA
C. Eat small meals very quickly
5. Which patient should use a long acting GLP-1RA according to KDIGO guidelines?
A. A patient achieving glycemic targets
B. A patient who cannot use SGLT-2i
C. A patient with significant hypotension
6. According to the AACE/ACE guidelines, when should clinicians consider an SGLT-2i?
A. first line
B. second line
C. third line
7. Which of the following statements about diabetic nephropathy is FALSE?
A. Symptoms of renal dysfunction include fatigue, weakness, shortness of breath, and itching.
B. Statins slow CKD progression and reduce all-cause cardiovascular disease mortality.
C. Controlling a patient’s BP with an ACEi or ARB is key to slowing CKD progression.
8. Patient AD has T2DM and CKD. She was prescribed dulaglutide. AD is also having a difficult time managing her postprandial glycemia. What recommendation can you make to her provider?
A. Replace with lixisenatide
B. Replace with semaglutide
C. Replace with weekly exenatide
9. Patient DN has T2DM and kidney function classified as G2A1. He is reluctant to continue treatment with canagliflozin ever since he heard it can cause symptomatic hypotension. What can you tell DN to help him adhere to treatment?
A. Symptomatic hypotension occurs more frequently in patients with eGFR < 60 mL/min/1.73m2.
B. Symptomatic hypotension occurs more frequently in patients with eGFR < 90 mL/min/1.73m2.
C. Symptomatic hypotension occurs more frequently in patients with eGFR > 60 mL/min/1.73m2.
10. What is the HbA1C target recommended by KDIGO guidelines to prevent or delay progression of the microvascular complications of diabetes?
A. Less than 8%
B. About 6.5%
C. About 7%
11. EM approaches the pharmacy counter to pick up her prescriptions for metformin, losartan, and furosemide. She also wants to purchase naproxen for headache. How can you help Emma with her OTC choice?
A. Recommend Emma try acetaminophen since naproxen is an NSAID.
B. Counsel Emma to use aspirin since it will not be absorbed systemically.
C. Advise Emma to try ibuprofen since it is a stronger NSAID than naproxen.
Pharmacy Technician Post Test (for viewing only)
Pharmacy Technician Post-test
After completing this continuing education activity, pharmacy technicians will be able to
• List the basic pathology and symptoms of renal disease and diabetes.
• Recall treatments used in patients who have comorbid renal disease and diabetes.
• Identify when to refer patients to the pharmacists for recommendations or referrals.
1. Which statement is TRUE about treatments used in patients who have comorbid renal disease and diabetes?
A. GLP-1RA increase blood pressure
B. SGLT-2i cause loss of muscle mass
C. SGLT-2i reduce CKD progression
2. A patient you know has T2DM approaches the counter and wants to purchase an OTC item. Which of the following medications would flag you to ask the pharmacist for a consultation?
A. Acetaminophen
B. Naproxen
C. Lidocaine
3. Which symptom corresponds with more than three quarters of kidney function lost?
A. Constipation
B. Cough
C. Itching
4. Which medication is an example of a short acting GLP1-RA ?
A. lixisenatide
B. semaglutide
C. dulaglutide
5. A patient is picking up her prescriptions for calcitriol, sevelamer carbonate, and losartan. The patient has a headache and is also purchasing OTC aspirin. What should you do?
A. Complete the transaction, aspirin is safe for the treatment of headaches in patients with advanced kidney disease.
B. Refer the patient to the pharmacist; aspirin is an NSAID that should not be taken by patients with advanced kidney disease.
C. Recommend the patient try ibuprofen because it is the best alternative for patients with advanced kidney disease.
6. What contributes to glomerular hyperfiltration in T2DM?
A. Hypertension
B. Diarrhea
C. Rhinorrhea
7. Patient KD approaches the pharmacy counter and asks you to explain what eGFR is and why it is important. What do you tell him?
A. eGFR is a direct measure of your glomerular filtration rate. It is important because it estimates the number of functioning nephrons in your kidneys.
B. eGFR is an estimation of your glomerular filtration rate. It is important because it estimates the number of functioning nephrons in your kidneys.
C. eGFR is the filter in your kidneys. It is important because it returns useful substances to blood, and waste and extra water becomes urine.
8. Which medication is a SGLT-2i?
A. metformin
B. exenatide
C. canagilflozin
9. What is an adverse event associated with SGLT-2i?
A. Genitourinary infections
B. Antibody formation
C. Gastrointestinal symptoms
10. What is a benefit of using GLP-1RA?
A. Weight loss of 3-5 kg
B. Lower HbA1C 2-2.5%
C. Weight loss of 1-2 kg
References
Full List of References
References
1. Benjamin N Gross, Vidhi Shah, and Michelle Z Farland, (2017), "Diabetes mellitus," PharmacotherapyFirst: A Multimedia Learning Resource. Accessed May 13, 2021. https://doi.org/10.21019/pharmacotherapyfirst.dm_overview
2. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-2045. doi:10.2215/CJN.11491116
3. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1-S115. doi:10.1016/j.kint.2020.06.019
4. Navaneethan SD, Zoungas S, Caramori ML, et al. Diabetes management in chronic kidney disease: synopsis of the 2020 KDIGO clinical practice guideline. Ann Intern Med. 2021;174(3):385-394. doi:10.7326/M20-5938
5. Famous People and Kidney Disease. Renal Support Network. Updated July 9, 2021. Accessed July 22, 2021. https://www.rsnhope.org/rsn-blog/famous-people-and-kidney-disease/
6. American Diabetes Association Diabetes Care 2020 Jan; 43(Supplement 1): S135-S151. https://doi.org/10.2337/dc20-S011
7. Wu B, Bell K, Stanford A, et al. Understanding CKD among patients with T2DM: prevalence, temporal trends, and treatment patterns-NHANES 2007-2012. BMJ Open Diabetes Res Care. 2016;4(1):e000154. doi:10.1136/bmjdrc-2015-000154
8. Chronic Kidney Disease Nutrition Management Training Program. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Updated September 2013. Accessed May 15, 2021. https://www.niddk.nih.gov/health-information/professionals/education-cme/management-training-program
9. Dowling TC. Evaluation of kidney function. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10e. McGraw-Hill; Accessed May 13, 2021. https://usjezproxy.usj.edu:2608/content.aspx?bookid=1861§ionid=134127006
10. Chronic Kidney Disease Management for Pharmacists. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Updated January 2020. Accessed May 15, 2021. https://www.niddk.nih.gov/health-information/professionals/education-cme/chronic-kidney-disease-management-pharmacists
11. Fatema K, Abedin Z, Mansur A, et al. Screening for chronic kidney diseases among an adult population. Saudi J Kidney Dis Transpl. 2013;24(3):534-541. doi:10.4103/1319-2442.111049
12. Hudson JQ, Wazny LD. Chronic kidney disease. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10e. McGraw-Hill; Accessed May 13, 2021. https://usj-ezproxy.usj.edu:2608/content.aspx?bookid=1861§ionid=134127380
13. Kidney Beginnings. American Association of Kidney Patients (AAKP). Published 2017. Accessed May 15, 2021. https://aakp.org/wp-content/uploads/2019/09/KB-Book_2017_FINAL_online_version.pdf
14. Chronic Kidney Disease (CKD). National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Updated October 2016. Accessed May 15, 2021. https://www.niddk.nih.gov/health-information/kidney-disease/chronic-kidney-disease-ckd/all-content
15. Cockcroft-Gault Formula. National Kidney Foundation. Updated 2021. Accessed September 20, 2021. https://www.kidney.org/professionals/kdoqi/gfr_calculatorcoc
16. Estimating Glomerular Filtration Rate. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Accessed September 20, 2021. https://www.niddk.nih.gov/health-information/professionals/clinical-tools-patient-management/kidney-disease/laboratory-evaluation/glomerular-filtration-rate/estimating
17. Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825-830. doi:10.7326/0003-4819-158-11-201306040-00007
18. Morris H, Mohan S. Using race in the estimation of glomerular filtration rates: time for a reversal?. Curr Opin Nephrol Hypertens. 2020;29(2):227-231. doi:10.1097/MNH.0000000000000587
19. Eneanya ND, Yang W, Reese PP. Reconsidering the Consequences of Using Race to Estimate Kidney Function. JAMA. 2019;322(2):113–114. doi:10.1001/jama.2019.5774
20. Delgado C, Baweja M, Crews D, et al. A Unifying Approach for GFR Estimation: Recommendations of the NKF-ASN Task Force on Reassessing the Inclusion of Race in Diagnosing Kidney Disease [published online ahead of print, 2021 Sep 23]. J Am Soc Nephrol. 2021;ASN.2021070988. doi:10.1681/ASN.2021070988
21. Munar MY, Singh H. Drug dosing adjustments in patients with chronic kidney disease. Am Fam Physician. 2007;75(10):1487-1496.
22. CKD & Drug Dosing: Information for Providers. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Updated April 2015. Accessed September 20, 2021. https://www.niddk.nih.gov/research-funding/research-programs/kidney-clinical-research-epidemiology/laboratory/ckd-drug-dosing-providers
23. KDIGO 2012 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney International Supplements. 2013;3(1):262-265. doi:10.1038/kisup.2013.30
24. Levey AS, Eckardt KU, Dorman NM, et al. Nomenclature for kidney function and disease: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int. 2020;97(6):1117-1129. doi:10.1016/j.kint.2020.02.010
25. Stage 5 of Chronic Kidney Disease (End Stage Renal Disease). Fresnius Kidney Care. Updated 2020. Accessed July 16, 2021. https://www.freseniuskidneycare.com/kidney-disease/stages/stage-5
26. Schreiner GE. How end-stage renal disease (ESRD)-medicare developed. Am J Kidney Dis. 2000;35(4 Suppl 1):S37-S44. doi:10.1016/s0272-6386(00)70229-0
27. Savage T, Browne T. Dialysis rationing and the just allocation of resources: a historical primer. J. Am. Soc. Nephrol. 2012;36(Winter 2012):37-42. Accessed July 22, 2021. https://www.kidney.org/sites/default/files/v36b_a4.pdf
28. Diabetic Kidney Disease. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Updated February 2017. Accessed May 15, 2021. https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/diabetic-kidney-disease
29. Helping Diabetes Educators Care for Patients with Kidney Disease. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Updated September 2014. Accessed May 15, 2021. https://www.niddk.nih.gov/health-information/professionals/education-cme/helping-diabetes-educators-kidney-disease
30. Bernstein KE, Ong FS, Blackwell WL, et al. A modern understanding of the traditional and nontraditional biological functions of angiotensin-converting enzyme [published correction appears in Pharmacol Rev. 2013;65(1):544]. Pharmacol Rev. 2012;65(1):1-46. Published 2012 Dec 20. doi:10.1124/pr.112.006809
31. Ruggenenti P, Perna A, Gherardi G, et al. Renoprotective properties of ACE-inhibitors in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 1999;354:359-364.
32. Lewis EJ., Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin-receptor antagonists irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;345:851-860.
33. Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001;345:861-869.
34. Hou FF, Zhang X, Zhang GH, et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 2006;354:131-140.
35. Beitelshees AL, Leslie BR, Taylor SI. Sodium-glucose cotransporter 2 inhibitors: a case study in translational research. Diabetes. 2019;68(6):1109-1120. doi:10.2337/dbi18-0006
36. Invokana. Prescribing information. Janssen Pharmaceuticals, Inc.; 2019. Accessed May 15, 2021. https://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/INVOKANA-pi.pdf
37. Farxiga. Prescribing information. AstraZeneca Pharmaceuticals LP; 2021. Accessed May 15, 2021. https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/0be9cb1b-3b33-41c7-bfc2-04c9f718e442/0be9cb1b-3b33-41c7-bfc2-04c9f718e442_viewable_rendition__v.pdf
38. Jardiance. Prescribing information. Boehringer Ingelheim Pharmaceuticals, Inc.; 2020. Accessed May 15, 2021. https://docs.boehringer-ingelheim.com/Prescribing%20Information/PIs/Jardiance/jardiance.pdf
39. Triplitt CL, Repas T, Alvarez C. Diabetes mellitus. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 10e. McGraw-Hill; Accessed May 18, 2021. https://usj-ezproxy.usj.edu:2608/content.aspx?bookid=1861§ionid=146065891
40. Steglatro. Prescriber information. Merck & Co., Inc,;2021. Accessed June 11, 2021. https://www.merck.com/product/usa/pi_circulars/s/steglatro/steglatro_pi.pdf
41. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295-2306. doi:10.1056/NEJMoa1811744
42. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al. Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020;383(15):1436-1446. doi:10.1056/NEJMoa2024816
43. Górriz JL, Soler MJ, Navarro-González JF, et al. GLP-1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020;9(4):947. doi:10.3390/jcm9040947
44. Drucker DJ, Habener JF, Holst JJ. Discovery, characterization, and clinical development of the glucagon-like peptides. J Clin Invest. 2017;127(12):4217-4227. doi:10.1172/JCI97233
45. Victoza. Prescribing information. Novo Norodisc; 2020. Accessed May 15, 2021. https://www.novo-pi.com/victoza.pdf
46. Trulicity. Prescribing information. Eli Lilly and Company; 2021. Accessed May 15, 2021. https://uspl.lilly.com/trulicity/trulicity.html#pi
47. Byetta. Prescribing information. AstraZeneca Pharmaceuticals LP; 2021. Accessed May 15, 2020. https://den8dhaj6zs0e.cloudfront.net/50fd68b9-106b-4550-b5d0-12b045f8b184/ce8afab9-2b45-436d-957c-a73978d09e93/ce8afab9-2b45-436d-957c-a73978d09e93_viewable_rendition __v.pdf
48. Ozempic. Prescribing information. Novo Norodisc; 2021. Accessed May 15, 2021. https://www.novo-pi.com/ozempic.pdf
49. Adlyxin. Prescribing information. Sanofi-aventis U.S. LLC;2019. Accessed May 15, 2021. https://products.sanofi.us/adlyxin/adlyxin.pdf
50. Rybelsus. Prescribing information. Novo Norodisc; 2021. Accessed May 15, 2021. https://www.novo-pi.com/rybelsus.pdf
51. Bydureon Bcise. Prescribing information. AstraZeneca Pharmaceuticals LP; 2020. Accessed May 15, 2021. https://www.azpicentral.com/bydureon_bcise/bydureon_bcise.pdf#page=1
52. Wieczorek-Surdacka E, Surdacki A, Świerszcz J, Chyrchel B. Novel antidiabetic drugs in diabetic kidney disease accompanying type 2 diabetes - a minireview. Folia Med Cracov. 2020;60(4):97-101
53. Handelsman Y, Bloomgarden ZT, Grunberger G, et al. American Association of Clinical Endocrinologists and American College of Endocrinology - Clinical practice guidelines for developing a diabetes mellitus comprehensive care plan - 2015. Endocr Pract. 2015;21 Suppl 1(Suppl 1):1-87. doi:10.4158/EP15672.GL
54. Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines [published correction appears in J Am Coll Cardiol. 2018 May 15;71(19):2275-2279]. J Am Coll Cardiol. 2018;71(19):e127-e248. doi:10.1016/j.jacc.2017.11.006
55. Counseling Patients on NSAID Use to Prevent Acute Kidney Injury. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Updated February 2020. Accessed May 15, 2021. https://www.niddk.nih.gov/health-information/professionals/education-cme/counseling-patients-nsaid-use
56. Pain Medicines (Analgesics). National Kidney Foundation Inc (NKF). Updated February 25, 2021. Accessed July 16, 2021. https://www.kidney.org/atoz/content/painmeds_analgesics
57. Pham PC, Khaing K, Sievers TM, et al. 2017 update on pain management in patients with chronic kidney disease. Clin Kidney J. 2017;10(5):688-697. doi:10.1093/ckj/sfx080
58. Jennifer Bailey, Asha Tata, Laura Waite, and Ryan G. D’Angelo, (2017), "Chronic kidney disease," PharmacotherapyFirst: A Multimedia Learning Resource. Accessed May 13, 2021. https://doi.org/10.21019/pharmacotherapyfirst.ckd_overview
59. Sick Day Plan. Kidney Health Australia. Updated May 2017. Accessed June 25, 2021. https://kidney.org.au/uploads/resources/Acute-Kidney-Injury-Sick-Day-Plan-for-healthcare-professionals-sheet.pdf
60. Chronic Kidney Disease in the United States, 2021. Centers for Disease Control and Prevention (CDC). Updated March 4, 2021. Accessed July 16, 2021. https://www.cdc.gov/kidneydisease/publications-resources/ckd-national-facts.html
61. Narva AS, Norton JM, Boulware LE. Educating patients about CKD: the path to self-management and patient-centered care. Clin J Am Soc Nephrol. 2016;11(4):694-703. doi:10.2215/CJN.07680715
62. Foucault-Fruchard L, Bizzoto L, Allemang-Trivalle A, Renoult-Pierre P, Antier D. Compared benefits of educational programs dedicated to diabetic patients with or without community pharmacist involvement. Prim Health Care Res Dev. 2020;21:e49. doi:10.1017/S1463423620000390
63. Jang SM, Cerulli J, Grabe DW, et al. NSAID-avoidance education in community pharmacies for patients at high risk for acute kidney injury, upstate New York, 2011. Prev Chronic Dis. 2014;11:E220. Published 2014 Dec 18. doi:10.5888/pcd11.140298
64. Zhu L, Fox A, Chan YC. Enhancing collaborative pharmaceutical care for patients with chronic kidney disease: survey of community pharmacists. Can J Hosp Pharm. 2014;67(4):268-273. doi:10.4212/cjhp.v67i4.1370
65. Kleinaki Z, Kapnisi S, Theodorelou-Charitou SA, Nikas IP, Paschou SA. Type 2 diabetes mellitus management in patients with chronic kidney disease: an update. Hormones (Athens). 2020;19(4):467-476. doi:10.1007/s42000-020-00212-y
66. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98(4):839-848. doi:10.1016/j.kint.2020.06.024
67. Koliaki C, Tentolouris A, Eleftheriadou I, Melidonis A, Dimitriadis G, Tentolouris N. Clinical management of diabetes mellitus in the era of COVID-19: practical issues, peculiarities and concerns. J Clin Med. 2020;9(7):2288. doi:10.3390/jcm9072288
68. New Data Alert: COVID-19 Brings Crisis of Access for Millions Living with Diabetes. American Diabetes Association (ADA). Published December 23, 2020. Accessed April 15, 2021. https://www.diabetes.org/newsroom/press-releases/COVID-19-brings-crisis-of-access-for-millions-living-with-diabetes
69. Xu Z, Wang Z, Wang S, et al. The impact of type 2 diabetes and its management on the prognosis of patients with severe COVID-19. J Diabetes. 2020;12(12):909-918. doi:10.1111/1753-0407.13084
70. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. COVID-19 Treatment Guidelines Panel. National Institutes of Health (NIH). Updated July 8, 2021. Accessed July 16, 2021. https://www.covid19treatmentguidelines.nih.gov/
71. Hassanein M, Radhakrishnan Y, Sedor J, et al. COVID-19 and the kidney. Cleve Clin J Med. 2020;87(10):619-631. doi:10.3949/ccjm.87a.20072