Learning Objectives
After completing this application-based continuing education activity, pharmacists and pharmacy techicians will be able to
| · Describe emerging information about monkeypox |
| · Use this information to answer patients’ questions |
Release Date:
Release Date: June 18, 2022
Expiration Date: June 18, 2025
Course Fee
FREE
ACPE UANs
Pharmacist: 0009-0000-22-046-H01-P
Pharmacy Technician: 0009-0000-22-046-H01-T
Session Codes
Pharmacist: 22YC46-PXK36
Pharmacy Technician: 22YC46-KPX88
Accreditation Hours
1.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-22-046-H01-P/T will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Pamela Sardo, PharmD
Sardo Solutions
Josephine, TX
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Dr. Sardo has worked for Rhythm Pharmaceuticals. All potential conflicts of interest have been mitigated.
ABSTRACT
Pharmacy professionals and paraprofessionals are receiving questions about monkeypox. Monkeypox is caused by the monkeypox virus, a member of the Orthopoxvirus genus. It is a viral disease that usually occurs in tropical rainforest areas of central and west Africa, but recently clinicians are diagnosing cases in other regions around the globe. Monkeypox is less contagious than smallpox and generally causes less severe illness. Monkeypox is transmitted to humans through close contact with an infected person or animal, or with material contaminated with the virus. The virus is transmitted from one person to another by contact with lesions, body fluids, respiratory droplets, and contaminated materials such as bedding. Usually, it presents clinically with fever, rash, and swollen lymph nodes and may lead to a range of medical complications. Monkeypox is typically a self-limiting disease with the symptoms lasting from two to four weeks. Severe and atypical cases can occur. Guidance from the Centers for Disease Control and Prevention and other health agencies is evolving. Treatment options for monkeypox may include smallpox vaccine, cidofovir, tecovirimat, and vaccinia immune globulin (VIG), among others.
CONTENT
Content
Introduction
Monkeypox is a rare virus believed to be transmitted to humans from animals and is endemic to Central and West Africa, typically near tropical rainforests.1 There are two clades (groups that share a common biological ancestor) of monkeypox virus: the West African clade and Congo Basin clade. More severe illness has been reported from the Congo Basin clade. Monkeypox is caused by monkeypox virus, a member of the Orthopoxvirus genus.2
The U.S. Centers for Disease Control and Prevention (CDC), in coordination with state and local officials, have initiated an emergency response to identify, monitor, and investigate monkeypox. This response includes a Health Alert Network (HAN), which is developing public health and clinical recommendations by developing protocols, medical guidance, and facilitating delivery of vaccine postexposure prophylaxis (PEP). The HAN also provides evolving education regarding antivirals that the U.S. government has stockpiled for preparedness and response purposes.2 Antivirals are discussed later in this continuing education activity.
Exposure to Monkeypox
Much like how we peel a banana to see what is underneath, let’s peel away the mystery of monkeypox exposure. Transmission of the monkeypox virus to humans can occur via animal bite or direct contact with the blood, meat, bodily fluids, cutaneous, or mucosal lesions of an infected animal. Human-to-human transmission by close direct contact and via exhaled large droplets is rare but can occur.3
Historically, human to-human transmission has been reported among household contacts and in other shared living quarters (e.g., in prisons). Transmission has also been reported in health care providers who have had close, sustained contact with a patient, objects, or materials that are likely to carry infection, such as bedding, clothes, utensils, and furniture. Touching and being face-to-face with someone who has symptoms are risk factors for exposure.2
Monkeypox can spread through close skin-to-skin contact during sex, including kissing, touching, and oral and penetrative sex with someone who has symptoms. Monkeypox rashes are sometimes found on genitals and in the mouth. Mouth-to-skin contact could cause transmission where skin or mouth lesions are present. It is currently unknown whether monkeypox can be spread through semen or vaginal fluids. People who have symptoms should avoid sexual contact with others. Until more is known, individuals should continue using condoms after they recover, and continue using condoms until we know more about this virus’s transmission.4
Although the evidence is limited and the risk of human-to-human transmission appears to be low, researchers report that infants and young children appear to be at the greatest risk of severe disease.5 The World Health Organization (WHO) reports that transmission from the mother to the fetus can occur via the placenta (which can lead to congenital monkeypox) or by close contact during and after birth.1
Symptom Presentation
Just like a variety of recipes include bananas, patients with monkeypox can experience a variety of symptoms. Patients with monkeypox typically experience a febrile prodrome five to 13 days after exposure (range = four to 17 days), which often includes lymphadenopathy (abnormal enlargement of the lymph nodes), malaise, headache, and muscle aches. The prodrome might vary depending on the nature of exposure. The onset of a characteristic skin rash generally occurs one to four days following the prodrome. The lesions are well defined and progress over time to scabs. The rash can be disseminated.2
The monkeypox rash progresses through sequential stages6:
- Macules—small spots, different in color from the surrounding tissue
- Papules—small circumscribed solid elevations on the skin
- Vesicles—small, circumscribed elevations on the skin containing fluid
- Pustules—small, circumscribed elevations of the skin containing purulent material
- Scabs—crust formed by coagulation of blood, pus, serum, or a combination
The rash can last up to a month.1 The rash can also be confused with other diseases that are more commonly encountered in clinical practice (e.g., secondary syphilis, herpes, and varicella zoster). Researchers report some patients are co-infected with monkeypox virus and other infectious agents (e.g., varicella zoster or syphilis). Patients with a characteristic rash should be advised to receive testing.2
Monkeypox’s clinical presentation is occasionally inconsistent. Some cases present classically, while other cases are atypical. Common symptoms include genital and peri-anal lesions, fever, swollen lymph nodes, and pain when swallowing. Oral sores remain a common feature in combination with fever and swollen lymph nodes. In some cases, pustules appear before classical symptoms (e.g., fever) and lesions appear at different stages. Anogenital rash (with vesicular, pustular, or ulcerated lesions) sometimes appears first without spreading to other parts of the body. This initial presentation of a genital or peri-anal rash suggests close physical contact as the likely route of transmission, such as during sexual contact.7
Pause and Ponder Question: How often should you ask patients with fever if they have also had close contact with someone with similar symptoms or a rash or headache?
Avoiding Monkeypox
Some learners may avoid eating bananas. Let’s avoid monkeypox. As of June 2022, clinicians in Europe, North America, South America, Africa, Asia, and Australia have reported monkeypox cases. Public health officials are cautioning travelers to avoid close contact with dead or live wild animals such as small rodents and monkeys. Travelers should avoid eating prepared meat from wild game or using products such as creams and lotions derived from wild animals in Africa, where cases of monkeypox are mainly found.8
Health care professionals should continue to watch for updates and evolving guidance as more information becomes known. The CDC raised its alert level for monkeypox to level 2. (see Figure 1) An example of evolving guidance is that, initially, the CDC recommended that people wear masks when traveling. A few days later, the advice regarding mask-wearing is no longer present on the CDC web sites. Thus, their advice evolved.2 Travel watch alert level 2 continues to encourage people to practice enhanced precaution measures, such as avoiding contact with visibly sick people and regularly washing hands.9
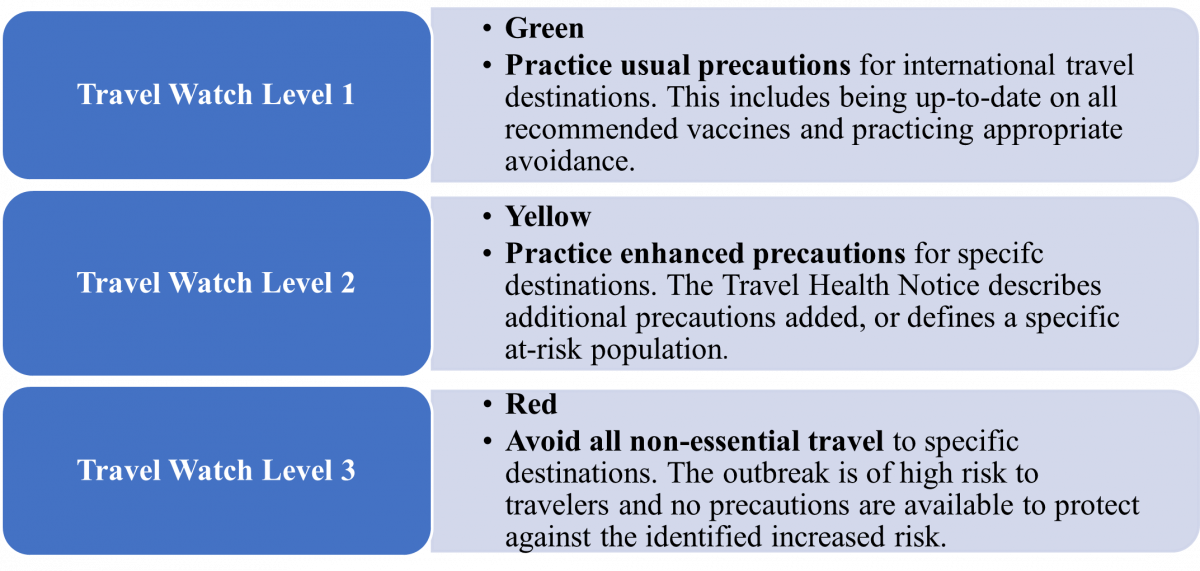
Figure 1. Centers for Disease Control and Prevention Travel Health Notices9
Researchers recommend the following measures to prevent infection with monkeypox2:
- Isolate ill people from uninfected people
- Practice good hand hygiene and use appropriate personal protective equipment to protect household members if ill or caring for ill persons at home (e.g., long sleeves and pants, and disposable gloves)
- Use an Environmental Protection Agency–registered disinfectant with emerging viral pathogens claim that is found on its List Q for disinfection of surfaces. Examples include hydrogen peroxide, isopropyl alcohol and citric acid among others.10 This list is available at https://www.epa.gov/pesticide-registration/disinfectants-emerging-viral-pathogens-evps-list-q
- Patients should also avoid contact with pets and other animals while infectious, because some mammals might be susceptible to monkeypox
- A person is considered infectious from the onset of illness until all lesions have crusted over, those crusts have separated, and a fresh layer of healthy skin has formed under the crust
Pause and Ponder Question: How can you make individuals in your practice setting more comfortable about the U.S. monkeypox outbreak?
Potential Health Impact
The WHO communicates that monkeypox is less contagious than smallpox and causes less severe illness. Monkeypox is usually a self-limiting condition with the symptoms lasting from two to four weeks.1
For individuals with monkeypox, isolation precautions should continue until all lesions have resolved, the scabs have fallen off, and a fresh layer of intact skin has formed. Patients who do not require hospitalization but remain potentially infectious should isolate at home. Clinicians should discontinue isolation only after consultation with the local or state health department.10
Beyond self-limiting cases, severe cases can occur and may lead to a range of medical complications.1 Conditions leading to hospitalization have included the need to provide adequate pain management and the need to treat secondary infections.7 Agencies are communicating monkeypox updates quickly, so many unknowns remain. Data reports of mortality are widely inconsistent so are not discussed in this manuscript.
Learners should refer to their states’ public health departments to determine if and how they should report suspected or confirmed monkeypox.
Pause and Ponder Question: The WHO is considering a name change for monkeypox. What do you believe would be a clinically accurate name?
APPROACH TO TREATMENT
Scientists say the risk of monkeypox becoming established in non-endemic countries is real. The WHO urges affected countries to make every effort to identify all cases and contacts to control this outbreak and prevent further spread. The FDA has approved a vaccine and has allowed expanded access for additional treatments for monkeypox, but these are in limited supply.11
The WHO is developing a coordination mechanism for the distribution of supplies based on public health need. The WHO does not recommend mass vaccination against monkeypox. In the few places where vaccines are available, they are being used to protect those who may be exposed, such as health workers and laboratory personnel.11
In general, some countries may consider post-exposure vaccination, ideally within four days of exposure, for higher-risk close contacts such as sexual partners, family members in the same household, and health workers.11 The CDC facilitates the availability of vaccine post-exposure prophylaxis (PEP) to contacts with high-risk exposures (e.g., unprotected contact with a patient’s skin or mucous membranes, lesion, or body fluids).10
PEP is not recommended for people with low or uncertain risk (e.g., health care providers entering a patient’s room without eye protection). Eligible intermediate- and high-risk contacts are offered PEP with2,12,13
- smallpox (vaccinia) vaccine, live (ACAM2000) or
- smallpox and monkeypox vaccine, live, nonreplicating (Jynneos) vaccines.
Researchers and regulatory authorities have not communicated whether optimal doses of expanded access investigational new drug (EA-IND) therapeutics for patients presenting with monkeypox will be consistently the same or different from doses for other indications. Clinicians should consult the full prescribing information for each therapeutic for comprehensive information, and pharmacy technicians should use the approved prescribing information to find information on storage conditions if necessary. Table 1 lists FDA-approved treatments for people presenting with monkeypox. It also describes therapeutics with EA-IND protocols for monkeypox or treatments undergoing further research.
Table 1. Approved and Investigational Therapeutics for Monkeypox12-17
| Generic Name (Brand Name; Manufacturer) | Current FDA approved indication |
| Smallpox (Vaccinia) Vaccine, Live (ACAM2000) | Vaccine indicated for active immunization against smallpox disease for persons determined to be at high risk for smallpox infection |
| Smallpox and Monkeypox Vaccine, Live, Non-replicating (Jynneos) | Vaccine indicated for prevention of smallpox and monkeypox disease in adults 18 years of age and older determined to be at high risk for smallpox or monkeypox infection |
| Tecovirimat (TPOXX)
|
Capsules and injection indicated for the treatment of human smallpox disease in adults and pediatric patients weighing ≥ 3 kg |
| Cidofovir (Vistide) | Infusion indicated for CMV retinitis in patients with acquired immunodeficiency syndrome |
| Brincidofovir (Tembexa) | Tablets and oral solution indicated for the treatment of human smallpox disease in adult and pediatric patients, including neonates |
| Vaccinia immune globulin (human) (Vigiv) | Intravenous immune globulin indicated for the treatment of complications due to vaccinia vaccination, including eczema vaccinatum, progressive vaccinia, severe generalized vaccinia, vaccinia infections in individuals who have skin conditions, aberrant infections induced by vaccinia virus |
Smallpox Vaccine
The smallpox live vaccine listed in Table 1 is licensed for immunization in people who are at least 18 years old and at high risk for smallpox infection. It is contraindicated in individuals with severe immunodeficiency. Select warnings include myocarditis, encephalitis, ocular vaccinia, infants younger than 12 months, and pregnancy. Common adverse events include injection site reactions, malaise, fatigue, fever and headache.12 It can be used in people exposed to monkeypox if it is used under an EA-IND protocol. The smallpox vaccine is not currently available to the general public. In the event of another outbreak of monkeypox in the U.S., CDC will establish guidelines explaining who should be vaccinated.18
Smallpox and Monkeypox Vaccine
The FDA has licensed smallpox and monkeypox vaccine to prevent infection with smallpox and monkeypox viruses. Anaphylactic reactions after dosing are possible. In smallpox vaccine-naïve healthy adults, the most common injection site reactions were pain, redness, swelling, induration, and itching. The most common systemic adverse reactions were muscle pain, headache, fatigue, nausea and chills.13
Data from Africa suggests that this vaccine is at least 85% effective in preventing monkeypox. Effectiveness against monkeypox was concluded from a clinical study on the immunogenicity and efficacy data from animal studies. Researchers believe that vaccination after a monkeypox exposure may help prevent the disease or make it less severe.18
Tecovirimat
The U.S. government stockpiles tecovirimat.18 Tecovirimat reduces the production and release of enveloped orthopoxvirus in vitro for seven monkeypox virus strains.19 Tecovirimat use for monkeypox is not FDA approved, but the CDC holds a non-research expanded access EA-IND protocol that allows its use for primary or early empiric treatment of monkeypox in adults and children of all ages. Clinical trials demonstrate the drug is safe with only minor side effects.18 The injection is contraindicated in severe renal impairment. Clinicians should monitor for hypoglycemic symptoms. Both the capsules and injection may cause headache.14
Cidofovir and Brincidofovir
Data is not available on the effectiveness of cidofovir or brincidofovir in treating human cases of monkeypox. Both have proven activity against poxviruses in in vitro and animal studies. It is unknown whether a person with severe monkeypox infection will benefit from treatment with either antiviral, although their use may be considered in such instances.18 The CDC holds an expanded access protocol for both products that allows use of stockpiled cidofovir for the treatment of orthopoxviruses (including monkeypox) in an outbreak. Brincidofovir may have a better safety profile than cidofovir. Serious renal toxicity or other adverse events have not been observed during treatment of cytomegalovirus infections with brincidofovir compared to treatment using cidofovir.18
Vaccinia Immune Globulin
Data is unavailable on the effectiveness of vaccinia immune globulin (VIG) in treatment of monkeypox virus infection. Use of this immune globulin is under an EA-IND. Proven benefit in the treatment of monkeypox is currently undetermined; however, healthcare providers may consider its use in severe cases. VIG can be considered for prophylactic use in an exposed person with severe immunodeficiency in T-cell function for which smallpox vaccination following exposure to monkeypox virus is contraindicated.18 A boxed warning indicates maltose in immune globulin products may give falsely high blood glucose levels in certain types of blood glucose testing systems. Additional warnings include renal dysfunction, thrombotic events, and infusion rate precautions. The most common adverse drug reactions are headache and nausea.
Pause and Ponder Question: What side effects have your patients reported after receiving a vaccine?
Ancillary Treatment Options, Supportive Care
Bananas are considered comfort food by many people. What comfort care can be recommended for patients suffering with monkeypox? Monkeypox can impact multiple systems and co-infections are possible. Soap and water, povidone-iodine, silver sulfadiazine and moist occlusive bandages promote healing at lesions sites. Adequate hydration and nutrition and protecting vulnerable anatomical sites such as the eyes and genitals are critical. Treatment for pain is recommended but specific analgesics have not been specified.20
Antipyretics can be used for fever. Antiemetics can be used for nausea. For ocular infection, topical application of trifluridine has been used to resolve symptoms and to attempt to prevent possible ocular scarring. Topical or oral antibiotics have also been used in combination either to treat bacterial infection or as prophylactic therapy. Bronchodilation, nebulizer, or suctioning is recommended if the respiratory tract is affected.20
CONCLUSION
Pharmacists are knowledgeable about many viruses and now are engaged in conversations involving monkeypox. The CDC urges health care providers in the United States to be alert for patients who have rash or illnesses consistent with monkeypox, regardless of a patient’s gender, sexual orientation, history of international travel, or specific risk factors for monkeypox. All health care providers should contact their local or state health department if they suspect a case of monkeypox.
Pharmacist Post Test (for viewing only)
Pharmacist Post-test
EDUCATIONAL OBJECTIVES
After participating in this activity pharmacists and pharmacy technicians will be able to:
● Describe emerging information about monkeypox
● Use this information to answer patients’ questions
1.Which of the following is TRUE about monkeypox?
A. Monkeypox is more contagious than smallpox
B. Monkeypox transmission by bodily fluids can occur
C. Monkeypox is only contagious for four days
2. Which of the following is important regarding transmission of monkeypox?
A. The population at greatest risk for severe disease are infants and young children
B. Sexually active monkeypox infected individuals can stop using a condom after six days
C. Monkeypox transmission by exhaled droplets is the most common method of spread
3. When Tom Smith is requesting a refill, he tells you that he recently heard that monkeypox evolves into syphilis. What is your reply?
A. Yes, monkeypox evolves to primary syphilis but not secondary syphilis
B. Testing is not required for genital rashes because the evolution occurs
C. They are separate conditions but some people may be co-infected
4. Sally Williams comes to you for her travel vaccines before travel to Africa to hunt. She shares that she loves feeding the monkeys there and asks about monkeypox risk.
A. Tell her to get vaccinated with cidofovir and take insect repellant
B. She should avoid contact with monkeys, hunting and eating game meat
C. Tell her PEP is recommended in uncertain risk when she returns
5. What is one monkeypox potential impact on health?
A. Patients not requiring hospitalization but who are potentially infectious should home isolate
B. Conditions leading to hospitalization have included the need to provide behavioral consult
C. Discontinuation of isolation should be determined in consultation with respiratory therapist
6. A second year medical school fellow asks you whether to include recommendations for monkeypox vaccination routinely for clinic patients for whom she is caring. What do you say?
A. Tell the fellow “yes” because WHO does recommend mass vaccination against monkeypox
B. Tell the fellow you can provide information on CDC post-exposure prophylaxis criteria for individuals with high-risk exposure
C. Tell the fellow “no,” but post-exposure vaccination, ideally within four weeks of exposure, may be considered
7. Which vaccine is indicated for prevention of monkeypox in adults 18 years of age or older who are determined to be at high risk for infection?
A. TPOXX
B. VIGIV
C. JYNNEOS
Links to LO#1; answer found in Table 1 discussion of prevention
Rationale: Answer A is incorrect because TPOXX is indicated for smallpox. Answer B is incorrect because VIGIV is an infused immune globulin for complications. Answer C is CORRECT as Jynneos is indicated for both smallpox and monkeypox.
8. Which pharmaceutical can be used in people exposed to monkeypox, if it is used under an expanded access investigational new drug protocol?
A. Smallpox vaccine
B. Pfizer/BioNTech vaccine
C. Afluria Quadrivalent
9. Which therapeutic is stockpiled by the U. S. government, has an EA-IND and was found to have in vitro activity against seven monkeypox strains?
A. Vaccinia immune globulin
B. Tecovirimat
C. Cidofovir
10. What is a fact about the smallpox and monkeypox vaccine (JYNNEOS)?
A. Anaphylactic reactions after dosing are possible
B. Africa data suggests only 50% effectiveness
C. A common adverse reaction was afibrillation
Pharmacy Technician Post Test (for viewing only)
Go Bananas: Peel Away the Unknowns of Monkeypox
Pharmacy Technician Post-test
EDUCATIONAL OBJECTIVES
After participating in this activity pharmacists and pharmacy technicians will be able to:
● Describe emerging information about monkeypox
● Use this information to answer patients’ questions
1. Which of the following is TRUE about monkeypox?
A. Monkeypox is more contagious than smallpox
B. Monkeypox transmission by bodily fluids can occur
C. Monkeypox is only contagious for four days
2. Which of the following is important regarding transmission of monkeypox?
A. The population at greatest risk for severe disease are infants and young children
B. Sexually active monkeypox infected individuals can stop using a condom after six days
C. Monkeypox transmission by exhaled droplets is the most common method of spread
3. When Tom Smith is requesting a refill, he tells you that he recently heard that monkeypox evolves into syphilis. What is your reply?
A. Yes, monkeypox evolves to primary syphilis but not secondary syphilis
B. Testing is not required for genital rashes because the evolution occurs
C. They are separate conditions but some people may be co-infected
4. Sally Williams comes to you for her travel vaccines before travel to Africa to hunt. She shares that she loves feeding the monkeys there and asks about monkeypox risk.
A. Tell her to get vaccinated with cidofovir and take insect repellant
B. She should avoid contact with monkeys, hunting and eating game meat
C. Tell her PEP is recommended in uncertain risk when she returns
5. What is one monkeypox potential impact on health?
A. Patients not requiring hospitalization but who are potentially infectious should home isolate
B. Conditions leading to hospitalization have included the need to provide behavioral consult
C. Discontinuation of isolation should be determined in consultation with respiratory therapist
6. A nurse from a physician’s office in the building next door stops by and asks you about storage conditions for three vaccines they plan to order for their clinic patients. What is the BEST answer?
A. Tell her that none of the vaccines used for monkeypox are commercially available
B. Pull the complete prescribing information for the vaccines in question
C. Say that you need to consult with the pharmacist and you will call her later
7. Which vaccine is indicated for prevention of monkeypox in adults 18 years of age or older who are determined to be at high risk for infection?
A. TPOXX
B. VIGIV
C. JYNNEOS
8. Which pharmaceutical can be used in people exposed to monkeypox if it is used under an expanded access investigational new drug protocol?
A. Smallpox vaccine
B. Pfizer/BioNTech vaccine
C. Afluria Quadrivalent
9. Which therapeutic is stockpiled by the U. S. government, has an EA-IND and was found to have in vitro activity against seven monkeypox strains?
A. Vaccinia immune globulin
B. Tecovirimat
C. Cidofovir
10. What is a fact about the smallpox and monkeypox vaccine (JYNNEOS)?
A. Anaphylactic reactions after dosing are possible
B. Africa data suggests only 50% effectiveness
C. A common adverse reaction was afibrillation
References
Full List of References
References
References
1. Monkeypox. World Health Organization. Updated May 2022. Accessed June 8, 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox
2. Minhaj F, Ogale Y, Whitehall F, et al. Monkeypox outbreak-nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022 Jun 10;71(23):764-769. doi: 10.15585/mmwr.mm7123e1
3. Monkeypox. Transmission. Centers for Disease Control and Prevention. Updated May 29, 2022. Accessed June 12, 2022. https://www.cdc.gov/poxvirus/monkeypox/transmission.html
4. Public health advice for gay, bisexual and other men who have sex with men on the recent outbreak of monkeypox. World Health Organization. May 2022. Accessed June 8, 2022. public-health-advice-for-msm-on-monkeypox-22-may-2022.pdf (who.int)
5. Khalil A, Samara A, O'Brien P, Morris E, Draycott T, Lees C, et al. Monkeypox and pregnancy: what do obstetricians need to know? Ultrasound Obstet Gynecol. 2022 Jun 2. doi: 10.1002/uog.24968. Epub ahead of print
6. Dirckx, J, ed. Stedman’s concise medical dictionary for the health professions.3rd edition. Williams and Wilkins; 1997
7. Multi-country monkeypox update. World Health Organization. Updated June 4, 2022. Accessed June 8, 2022 Multi-country monkeypox outbreak: situation update (who.int)
8. Travel Health Notices. Centers for Disease Control and Prevention. Updated June 8, 2022. Accessed June 8, 2022. https://wwwnc.cdc.gov/travel/notices
9. Disinfectants for emerging viral pathogens. US Environmental Protection Agency. Updated June 2, 2022. Accessed June 12, 2022. https://www.epa.gov/pesticide-registration/disinfectants-emergingviral-pathogens-evps-list-q
10. Monkeypox. Duration of isolation procedures. Centers for Disease Control and Prevention. Updated May 31, 2022. Accessed June 12, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/isolation-procedures.html
11. WHO Director-General’s opening remarks at the COVID-19 media briefing-8 June 2022. World Health Organization. June 8, 2022. Accessed June 12, 2022. WHO Director-General's opening remarks at the COVID-19 media briefing – 8 June 2022
12. ACAM2000. Prescribing information. Emergent BioSolutions, Inc.; 2007. Accessed June 9, 2022
13. Jynneos. Prescribing information. Bavarian Nordic A/S; June 2021. Accessed June 8, 2022
14. Tpoxx. Prescribing information. Siga Technologies; May 2022. Accessed June 7, 2022.
15. Vistide. Prescribing information. Gilead Sciences, Inc. September 2000. Accessed June 8, 2022
16. Tembexa. Prescribing information. Chimerix, Inc.; June 2021. Accessed June 7, 2022
17. VIGIV. Prescribing information. Cangene Corporation. January 2010. Accessed June 12, 2022
18. Monkeypox. Treatment. Centers for Disease Control and Prevention. Updated June 9, 2022. Accessed June 9, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
19. Smith S, Olson V, Karem K, et al. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob Agents Chemother. 2009;53(3):1007–1012
20 Reynolds M, McCollum A, Nguete B, Shongo Lushima R, Peterson B. Improving the carand treatment of monkeypox patients in low resource settings: applying evidence from contemporary biomedical and smallpox biodefense research. Viruses. 2017; 9(12):380 doi: 10.3390/v9120380
