Learning Objectives
After completing this application-based continuing education activity, pharmacists and technicians will be able to
| 1. Describe the prevalence, pathophysiology, and prognosis of hepatorenal syndrome (HRS) |
| 2. Explain updated guidelines for diagnosis and treatment of HRS |
| 3. Discuss current and emerging therapies for HRS |
| 4. Identify the role of pharmacists and pharmacy technicians in HRS treatment |

Release Date: August 15, 2023
Expiration Date: August 15, 2025
Course Fee
Pharmacists: FREE
Pharmacy Technicians: FREE
This CE was funded by: Mallinckrodt
ACPE UANs
Pharmacist: 0009-0000-23-029-H01-P
Pharmacy Technician: 0009-0000-23-029-H01-T
Session Codes
Pharmacist: 23YC29-HPX34
Pharmacy Technician: 23YC29-XPX38
Accreditation Hours
2.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-23-029-H01-P/T will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Rachel Eyeler, PharmD, BCPS
Adjunct Clinical Professor
UConn School of Pharmacy
Storrs, CT
Nicole A. Pilch, PharmD, BCPS
Associate Professor Department of Pharmacy and Clinical Sciences
Medical University of South Carolina
Charleston, SC
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Drs. Eyeler and Pilch do not have any relationships with ineligible companies.
ABSTRACT
Hepatorenal syndrome (HRS) is a specific type of kidney injury unique to patients with end stage liver disease, also known as cirrhosis. Patients with cirrhosis have scarred, stiff livers in which blood cannot flow through easily. Portal hypertension changes blood flow resulting in several consequences: ascites, esophageal varices, and HRS. The American Association for the Study of Liver Diseases guidelines describe two distinct forms of HRS. Therapies such as volume resuscitation (e.g., with crystalloids or albumin) and vasoconstrictors (e.g., norepinephrine or terlipressin) focus on restoring blood flow to the kidneys before they are irreparably injured. Sometimes, clinicians must select therapies based on availability of intensive care unit beds and monitoring equipment. Clinicians also need to consider factors when patients leave the hospital and are discharged to home. Pharmacists and pharmacy technicians who are familiar with the basics of HRS can help clinicians make appropriate choices, counsel patients thoroughly, and contribute to better patient outcomes.
CONTENT
Content
INTRODUCTION
Hepatorenal syndrome (HRS), a type of kidney injury unique to patients with advanced liver disease, carries a grim prognosis. Therapies such as volume resuscitation (e.g., crystalloids or albumin) and vasoconstrictors (e.g., norepinephrine or terlipressin) focus on restoring blood flow to the kidneys before they are irreparably injured. Pharmacists and pharmacy technicians can play a crucial role in helping select and monitor therapies for treatment of this syndrome, and perhaps more importantly, by helping patients avoid developing HRS in the first place. By educating patients to avoid certain over the counter (OTC) medications that can worsen the condition (e.g., non-steroidal anti-inflammatory drugs [NSAIDs]), and teaching patients to monitor their diuretic use by weighing themselves daily and monitoring their blood pressure, engaged pharmacists and pharmacy technicians can make a large difference in their patients’ clinical outcomes.
HRS: A Complication of Cirrhosis
Cirrhosis, an advanced state of liver disease, is increasingly common and an important cause of mortality.1 Globally, in 2017 the estimated incidence of people living with compensated cirrhosis was 112 million. In 2019, cirrhosis was associated with 2.4% of deaths worldwide.2 Classically, cirrhosis in developed countries is most commonly due to hepatitis C infection and alcohol misuse.1 However, over the past decade, the incidence of non-alcoholic fatty liver disease (NAFLD) has increased dramatically with improvement in diagnostic criteria and screening. At the same time, treatment improvements for hepatitis B and C infections have decreased viral hepatitis-related deaths in some areas of the world. The COVID-19 pandemic has also had significant impact, with data collected from multiple countries showing a substantial increase in alcohol consumption, and an increase in alcohol-associated cirrhosis deaths.2,3 Testing and treatment rates for hepatitis B and C declined internationally between January 2019 and December 2020,4 and treatment delays are predicted to lead to excess liver-related deaths.2,5
PAUSE AND PONDER: How will the new hepatitis C direct-acting antivirals (e.g., elbasvir, glecaprevir, ledipasvir, pibrentasvir, sofosbuvir, velpatasvir, and voxilaprevir) change the landscape of HRS?
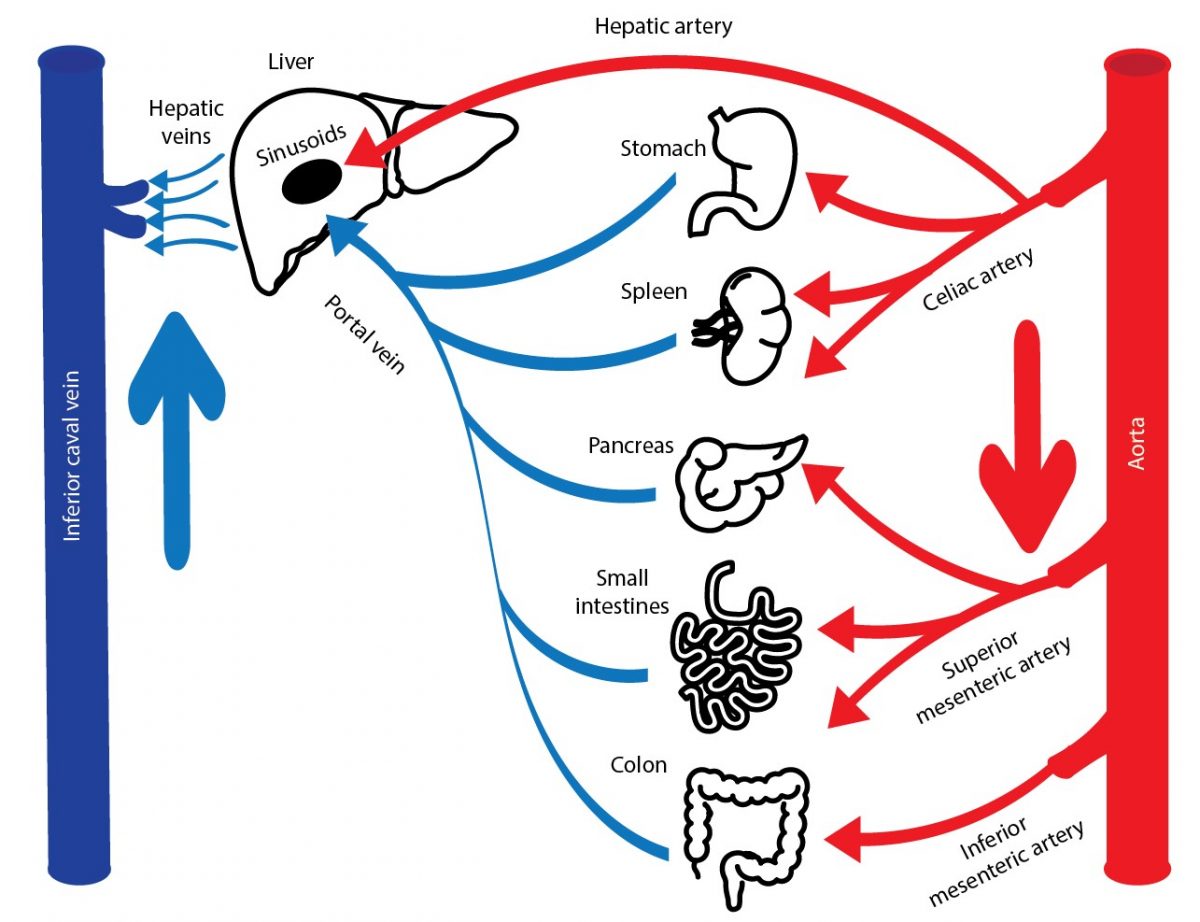
Although the causes of cirrhosis may be shifting, the transition from chronic liver disease to cirrhosis is generally the same. Chronic inflammation of the liver leads to fibrosis and scarring, causing structural and hemodynamic changes within the liver (Figure 1). One of the main consequences is development of portal hypertension. Portal hypertension results because the scarring of the liver makes it more difficult for blood to flow through it, leading to increased blood pressure in the portal vein as blood is delivered from the splanchnic organs (stomach, small intestine, colon, pancreas, and spleen) (Figure 2). The obstruction of blood flow through the portal vein additionally results in dilation of the splanchnic circulation as a compensatory mechanism aimed at restoring blood flow. In turn, increased blood flow to the splanchnic circulation worsens portal hypertension.1
Figure 1. Stages of Liver Disease.
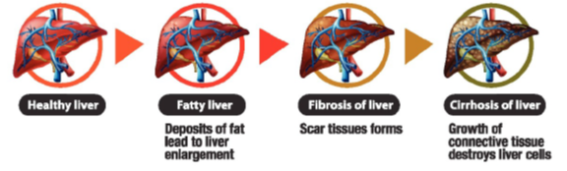
Figure 2. The splanchnic circulation. The splanchnic circulation describes blood flow to the abdominal organs. Blood from these “splanchnic” organs is delivered to the portal vein, and accounts for the majority of the blood flow to be processed by the liver.
Pathophysiology of HRS
Portal hypertension and the changes in blood flow that result are the main drivers of several consequences of cirrhosis. These complications include the development of ascites, espophageal varices, and HRS.
Ascites is fluid accumulation in the peritoneal cavity that commonly appears as abdominal swelling or bloat. A patient with significant ascites will often test positive for a “fluid wave.” That is, when a patient is lying flat and someone applies pressure to the abdominal midline, a clinician can tap one flank sharply, and an impulse or “shock wave” will travel through the fluid in the abdomen. The clinician will be able to be feel the tap on the other side.
Esophageal varices are enlarged veins in the esophagus that can lead to bleeding that commonly presents as “coffee ground” looking emesis (or vomitus) that is a result of the blood being digested in the stomach then regurgitated through the esophagus. In the case of HRS, the portal hypertension and splanchnic vessel dilation mean that blood tends to pool in the splanchnic circulation, decreasing effective arterial volume (i.e., a decreased amount of blood effectively perfusing organ tissue, including the kidneys). Additionally, the body activates various compensatory mechanisms aimed at increasing blood volume (e.g., the renin-angiotensin system and the sympathetic nervous system). This action is an attempt to restore effective blood volume, which leads to vasoconstriction of the kidney arterioles and further hypoperfusion of the kidney (Figure 3).6
Figure 3. Changes in blood flow with cirrhosis. The image on the left represents normal splanchnic and portal blood flow. The image on the right shows blood flow to a cirrhotic liver. Blood from the splanchnic organs meets increased resistance in the portal vein, leading to portal hypertension. The splanchnic arteries vasodilate which worsens portal hypertension and leads to decreased blood flow to the kidneys.
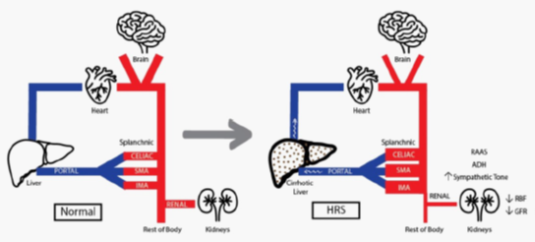
ABBREVIATIONS: ADH: antidiuretic hormone, IMA: inferior mesenteric artery, RAAS: renal angiotensin aldosterone system, RBF: renal blood flow, GFR: glomerular filtration rate, SMA: superior mesenteric artery
Prevalence and Prognosis
HRS is a type of acute kidney injury (AKI) that is unique to patients with decompensated cirrhosis. HRS occurs in the absence of hypovolemia or any structural changes to the kidney—in fact, the kidneys often function normally following liver transplantation.7 HRS is common in patients with cirrhosis, and risk of development increases as the severity of cirrhosis and the duration with which the patient has had it increase. In one study of patients with cirrhosis and ascites, the incidence of HRS increased from 18% at one year to 39% after five years.8 The development of HRS is unfortunately associated with a very poor prognosis, and often the only way to reverse the kidney failure is to receive a liver transplant.7
Classification and Diagnosis
Two distinct forms of HRS have been described. According to the American Association for the Study of Liver Diseases (AASLD) guidelines9
- Type 1 HRS is a rapid increase in creatinine (0.3 mg/dL or greater) within 48 hours or an increase in serum creatinine to levels that are at least 50% higher than the most recent baseline value measured within three months. It often has a precipitating factor, such as a bacterial infection, gastrointestinal bleeding, or over-diuresis. This type of HRS is more common and more severe, making up 75% of cases and having a median survival of one month.
- Type 2 HRS takes a longer time to develop and is defined as an estimated glomerular filtration rate of less than 60 mL/minute/1.73m2 for three months or more in the absence of other (structural) causes. This is the same definition used for all patients with chronic kidney disease (CKD). Type 2 HRS often co-occurs with other complications of cirrhosis (i.e., refractory ascites) and has a median survival of about seven months.8
In recent years the nomenclature has been updated so that type 1 HRS is referred to as HRS-AKI and type 2 is called HRS-CKD.6,9
Sometimes it is hard to know that a patient with end-stage liver disease is in kidney failure. These patients may have decreased muscle mass, are prone to malnutrition, and may take diuretics to control volume status. All three of those factors make serum creatinine an unreliable surrogate measure of kidney function.10
In patients with cirrhosis and ascites who meet the criteria for AKI, the diagnosis of HRS-AKI becomes one of exclusion. Clinicians must attempt to rule out hypovolemia, shock, medication-induced AKI, and structural kidney injury. In the absence of these alternative causes for AKI, a diagnosis of HRS-AKI can be made, and treatment commenced as soon as possible, as early intervention is key to decreasing mortality.9
Sidebar: Why is serum creatinine unreliable in advanced liver disease?
In clinical practice, clinicians often estimate kidney function by measuring a patient’s serum creatinine and inputting the value into a kidney function estimating equation. Creatinine is a byproduct of creatine, an amino acid produced by the liver and released into the circulation to reach target tissues, such as muscle. Creatinine is released during normal muscle metabolism. It is used in kidney function estimates because the glomerulus filters it freely, and so theoretically the rate at which the kidneys clear creatinine should be similar to the glomerular filtration rate itself.
However, a patient’s serum creatinine value is not affected by glomerular filtration rate alone. A malfunctioning liver may produce lower amounts of creatinine’s precursor, creatine. Additionally, patients with cirrhosis may have decreased oral intake due to nausea, ascites, and/or ongoing alcohol use. This means they consume less creatine is consumed from the diet as well. Finally, since creatinine is a product of muscle tissue breakdown and patients with cirrhosis tend to have significantly reduced muscle mass, they may generate less creatinine from the creatine. The end result is a serum creatinine that is normal or even lower than that seen in healthy individuals.
Hence, kidney function estimates that rely on creatinine tend to overestimate kidney function in these patients and even small absolute increases in creatinine could represent an acute kidney injury.
PAUSE AND PONDER: Is there a better way to measure true kidney function in patients with end-stage liver disease?
Current and Emerging Therapies for HRS
Therapies used to treat HRS aim at removing the precipitating factor and increasing blood flow to the kidneys. First, clinicians must identify and treat the potential etiology leading to the decline in kidney function (e.g., antibiotic therapy in the treatment of an infection of ascites fluid called spontaneous bacterial peritonitis [SBP], proton pump inhibitors, and endoscopic intervention to stop a gastrointestinal bleed). Removing the cause is one of the most important factors in ensuring that the change in kidney function is not permanent.
Fundamentally the kidney receives insufficient blood flow secondary to a decrease in effective arterial blood flow, so initial therapy’s main goal is to improve the patient’s mean arterial pressure as soon as possible. The most common goal cited is to increase the patient’s mean arterial pressure (MAP) to greater than 65 mmHg to improve perfusion to target end organs, specifically the kidney.11,12 The goal is to improve kidney function and give the patient additional time to secure a liver transplant or stabilize the end stage disease and decrease mortality. This continuing education activity will review the agents used to improve kidney function in the setting of HRS. Table 1 summarizes guideline recommendations for initial management of patients with HRS-AKI.
| Table 1. Summary of Society Guidelines for Initial Management of HRS-AKI in the ICU9,22,23,32,33 | ||||
| Society | HRS-AKI Definition | Volume expander | Vasopressor of choice | Target |
| American Association for the Surgery of Trauma 2022 | Increase in SCr > 0.3 mg/dL within 48 hours or > 50% increase in SCr in preceding 7 days in patients with cirrhosis/ascites without another cause | Lactate ringers or Plasmalyte over Normal Saline; albumin 20-25% 1 gm/kg/day x 48 hours | Norepinephrine, Terlipressin | MAP > 65 mmHg, increased urine output |
| American Association for the Study of Liver Disease 2021 | Increase in SCr > 0.3 mg/dL within 48 hours or > 50% increase in SCr in preceding 7 days in patients with cirrhosis/ascites without another cause; use SCr values for the last 3 months prior to event to evaluate baseline | Albumin 1 gm/kg on day 1, then 40 to 50 gm daily while receiving vasopressor therapy | Terlipressin,* Norepinephrine 0.5 mg/h ; max 3 mg/h | Increase MAP by at least 10 mmHg above pre-treatment baseline or urine output >200 mL over 4 hours; albumin to maintain CVP between 4-10 mmHg;
Continue treatment until SCr back to baseline up to 14 days; if SCr remains at or above pre-treatment values after 96 hours stop vasopressor therapy |
| European Association for the Study of the Liver 2010 | Kidney failure in the setting of liver disease unexplained by another cause | Albumin 1 gm/kg/day (max 100 gm/day) | Terlipressin 1 mg every 4 to 6 hours in combination with albumin, if SCr does not improve by at least 30% in 72 hours increase dose to 2 mg every 4 hours | Increase in MAP by at least 5 mmHg by day 3 |
| American Gastroenterological Association 2022 | Increase in SCr > 0.3 mg/dL within 48 hours or > 50% from baseline or urine output is <0.5 mL/kg/hr for > 6 hours | Albumin 1 gm/kg/day x 48 hours, if no improvement continue 1 gm/kg x 1 day then 20 to 40 gm daily while receiving vasopressor therapy | Terlipressin 1 mg every 4 to 6 hours; increase to 2 mg every 4 to 6 hours if reduction in SCr < 25% by day 3 if available up to 14 days, alternative norepinephrine or midodrine/octreotide | Increase MAP by at least 10 mmHg or urine output by at least 50 mL/h for at least 2 hours, maintain priority for liver transplant if survive |
| *not approved in US at the time of guideline construction
|
||||
| ABBREVIATIONS: CVP = central venous pressure; MAP = mean arterial pressure; SCr = serum creatinine | ||||
PAUSE AND PONDER: When might these interventions be considered “too late?” What would you do then?
Crystalloids
Initial evaluation of patients includes an assessment of their effective arterial blood volume status. Early cessation of home medications that impact blood pressure or volume status, such as diuretics (e.g., spironolactone, furosemide) will allow a more accurate determination. If the patient’s effective arterial blood volume is not optimized, inadequate blood flow to the kidney can precipitate acute kidney injury in the setting of cirrhosis.
Crystalloids, such as normal saline and Lactated Ringer’s, stay within the intravascular space and provide appropriate initial fluid replacement. However, clinicians must provide fluid replacement (also referred to in this case as fluid resuscitation) carefully and with appropriate monitoring as patients can become volume overloaded. Patients with end stage liver disease have low albumin levels and tend to lack the oncotic pressure (a type of osmotic pressure induced by plasma proteins, especially albumin) required to keep crystalloids within the intravascular space. Even minimal resuscitation can produce significant peripheral edema and pulmonary edema, and worsen ascites.13
Pharmacists should provide support in appropriate monitoring of volume status and ensuring serum electrolytes are frequently obtained. Fluids may need to be stopped abruptly in response to volume overload to prevent hypervolemic hyponatremia (low sodium levels).14 Appropriate understanding of the patient’s intravascular volume status will determine if using albumin during resuscitation is appropriate.
Albumin
Patients with end-stage liver disease typically have reduced or low albumin levels because the liver is no longer able to manufacture these proteins. Reduced albumin decreases the circulating oncotic pressure which yields fluid leakage from blood vessels into other areas of the body (e.g., peritoneal space), reducing the arterial blood volume going to the kidney. This becomes especially apparent when a precipitating event such as a hemorrhage or infection occurs, which further decreases circulating blood volume. Clinicians often administer concentrated albumin (e.g., 25% or 25 grams/100 mL) every six to eight hours to increase the intravascular circulating blood volume. Albumin allows fluid to move from the interstitial spaces back into the blood stream and keeps exogenously administered crystalloids in the vessels, thereby increasing blood flow to the kidneys.12 Clinicians should reserve concentrated albumin for patients with baseline low serum albumin (e.g., less than 3 mg/dL) who are also volume overloaded and limit them to just the amount that restores hemodynamic stability.15
Patients who have inadequate total body volume or those who have capillary leak (e.g., septic shock) may benefit from less concentrated (e.g., 5%) albumin infusions. A recent single center open-label, randomized study evaluated hypotensive patients with end-stage liver disease and compared volume replacement with albumin to normal saline.16 The primary outcome was to determine which approach could reverse a mean arterial pressure less than 65 mmHg more effectively within the first hours of resuscitation. Of note this trial excluded patients who needed immediate interventions, such as variceal bleed or vasopressor agents.17 The researchers randomized patients to receive 250 mL of 5% albumin over 30 minutes followed by 50 mL/hr for three hours or normal saline 30 mL/kg over 15 to 30 minutes followed by 100 mL/h over three hours. Albumin was more effective than normal saline in improving mean arterial pressure above 65 mmHg in the first hour (25.3% albumin vs 14.9% normal saline, p = 0.03) of resuscitation. The benefit continued over the next three hours (p < 0.001) and survival was also better in patients resuscitated with albumin than those treated with saline (43.5% vs 38.3%).16,18 The researchers note that results are predicated on appropriate management of the underlying causes of hypotension (i.e., sepsis).
Albumin is expensive, can be and has been subject to shortages. It should be used with stewardship in end stage liver disease and HRS; however, the evidence for benefit is robust especially when combined with other modalities.16 It is important to understand the patient’s volume status and hemodynamic goals to select the appropriate concentration and frequency.19,20 The AASLD Guidelines for the Diagnosis, Evaluation and Management of Ascites, Spontaneous Bacterial Peritonitis and HRS suggest that patients who present with HRS should receive 1 gram/kg albumin on day 1 and then 40 to 50 grams per day until kidney function improves and other therapies are no longer needed.9,15 The daily dose may be reduced (e.g., 20 to 40 grams/day) if given in combination with vasopressor agents with a goal to maintain adequate volume. Clinicians sometimes use a surrogate measure of volume using a central venous catheter to measure central venous pressure (CVP), which reflects the amount of blood in the patient’s anterior vena cava and venous tone. In this case, a CVP goal between 10 and 15 mmHg is targeted.10,21 Unfortunately CVP can be unreliable when ascites is present and clinicians may need to employ other invasive methods along with close monitoring for the development of pulmonary edema.9,22,23
Ensuring albumin is available for patients with HRS is necessary. Some ways to aid centers in managing their supplies when shortages occur include limiting scheduled orders to 24 hours (e.g., 1 gram of 25% every 8 hours for 24 hours). Limiting “evergreen” orders to 24 hours will ensure clinicians assess patients appropriately before administering additional albumin and may prevent unappreciated volume overload. Also, standardized order sets will prevent use of partial vials, larger than needed vials (e.g., 250 mL or 500 mL) and inappropriate ordering of the incorrect concentration (e.g., 5% versus 25%). Teaching hospitals may also benefit from limiting albumin orders to certain clinical situations or diagnoses to avoid ubiquitous use for volume resuscitation in patients (e.g., trauma) who can be resuscitated with crystalloid.
Vasopressors
Vasopressors are given in combination with resuscitation, specifically albumin. The exogenous albumin facilitates adequate oncotic pressure to keep fluid in the vasculature, allowing vasopressors to constrict the vessels, increase mean arterial pressure, and supply fluid to the kidney. Vasopressors will be ineffective and can make kidney function worse if fluid in the vasculature is insufficient. Therefore, prescribers should only institute vasopressors along with or after volume resuscitation. The main adverse effects associated with any vasopressor therapy are related to ischemia (poor blood flow) in the peripheral limbs/tissues (e.g., fingertips, skin), gastrointestinal tract, or heart.9 Limited head-to-head trials exist to identify which agent or combination is the most effective in reversing HRS beyond early implementation of therapy in combination with albumin volume expansion. Table 2 summarizes the pros, cons, and considerations related to vasopressor agents used in the treatment of HRS.
| Table 2. Vasopressor Agents Pros, Cons and Considerations | |||
| Medication | Pros | Cons | Considerations |
| Norepinephrine | Frequently used in the ICU setting, team comfort with monitoring for adverse effects and ease/experience with titration | May be less effective in hypothermia, pH dysregulation, continuous infusion | May require ICU setting, especially for acute titration; may require a central line |
| Terlipressin | Does not require a central line, or continuous infusion | Requires additional monitoring for ischemia which may require ICU level care to ensure safety | Requires monitoring for ischemic events |
| Octreotide | Can be given outside the ICU | Slow response, IV or subcutaneous administration | May be continued as an outpatient |
| Midodrine | Available as an oral agent, can be given outside the ICU | Slow response, only available as an oral agent; frequency of dosing | May be provided on discharge to help maintain blood pressure in the setting of hypotension |
ABBREVIATIONS: ICU = intensive care unit; IV = intravenous
Norepinephrine
Norepinephrine is an exogenous catecholamine that targets alpha-1-adrenergic receptors which helps improve peripheral vascular resistance.24 Norepinephrine has been used consistently in the United States (U.S.) for many years and has been the agent of choice until the recent approval of terlipressin. Norepinephrine’s limitation is that it can be less effective, as are other catecholamines, if patients have temperature or pH dysregulation. Appropriate resuscitation and correction of these variables can improve norepinephrine’s efficacy. A meta-analysis comparing the effectiveness of norepinephrine and terlipressin suggests that norepinephrine is as effective in increasing mean arterial pressure and reversal of kidney dysfunction.24 The most frequent doses of norepinephrine cited in terlipressin head-to-head trials were between 0.5 to 3 mg/hour and/or 0.05 to 0.7 mcg/kg/minute titrated to increase mean arterial pressure 10 mmHg above baseline or increasing urine output to more than 200 mL/hour.24 Clinicians must monitor norepinephrine administration carefully to prevent complications of vasoconstriction, such as cardiac or digital ischemia and therefore it is often restricted to the intensive care unit (ICU).24
Terlipressin
Terlipressin is a prohormone of lysine-vasopressin, causing extended release of lysine-vasopressin and activation of V1 and V2 receptors allowing intermittent administration.24 V1 receptors are predominantly located in the smooth muscles of the arterial vasculature in the splanchnic region. Activating V1 receptors constricts the splanchnic vessels (reducing delivery of blood flow to the portal vein, lowering portal pressure) which subsequently may improve blood flow to kidneys. Additionally, activation of the V2 receptors causes reabsorption of water in the kidney.24,25 Terlipressin has been evaluated in several prospective, placebo-controlled clinical trials evaluating its efficacy in improving kidney function in HRS.18 Specifically, a recent prospective, randomized, double-blind controlled trial evaluated the effectiveness of terlipressin against placebo in combination with albumin in reversing HRS-AKI.25 Terlipressin was more effective than placebo in reversing HRS (32% vs 17%, p < 0.006), but did yield more respiratory failure.25 This trial was an impetus for U.S. Food and Drug Administration (FDA) approval of terlipressin in 2023.
The FDA approved terlipressin for rapid reduction in kidney function in the setting of cirrhosis with no other etiology, or reversal of HRS at a dose of 1 mg administered by intravenous bolus every six hours.10 If the patient’s serum creatinine fails to improve or increases within the first 96 hours then prescribers should discontinue terlipressin. If improvement is marginal (e.g., less than 30% from baseline) the dose can be increased to 2 mg every six hours.10 Therapy should be continued until the patient’s serum creatinine is 1.5 mg/dL or less for two days or a maximum of 14 days. Prescribers should use terlipressin with caution in patients with a history of ischemic conditions (e.g., cardiac, mesenteric).10 Terlipressin should not be used in patients who have a serum creatinine exceeding 5 mg/dL, in patients who are hypoxic (SpO2 less than 90%), or in patients who develop ischemia.10,23
Terlipressin is often given outside the ICU and does not need continuous cardiac monitoring, which may make it desirable for longer term administration.10 Initial clinical trials compared norepinephrine continuous infusion (1 mcg/kg/minute increased every four hours to increase MAP by 10 mmHg) to terlipressin (1 mg every four hours; increased to 2 mg every four hours after three days) combined with albumin to maintain a CVP between 10 and 15 mmHg. These trials defined a complete response as an improvement in serum creatinine by at least 30% from baseline within 14 days of therapy. There was no difference in responders between norepinephrine and terlipressin (70% and 83%).21,26,27 Therefore terlipressin’s benefit may lie in its intermittent dosing and ability to be administered outside the ICU.
Terlipressin’s most common adverse reactions (≥10%) include abdominal pain, nausea, respiratory failure, diarrhea, and dyspnea. Terlipressin does have some additional considerations. It also has a boxed warning for possible serious or fatal respiratory failure, and clinicians need to monitor patients’ oxygen saturation carefully.28
Terlipressin is supplied as a single dose 0.85 mg vial that must be stored under refrigeration and protected from light. Vials are reconstituted with 5 mL of sodium chloride and if not used, must be refrigerated and expire after 48 hours. The initial dose based on the approved labeling is one vial (0.85 mg) every six hours; which can be increased to two vials (1.7 mg) every six hours.28
Midodrine and Octreotide
Midodrine and octreotide in combination have been a staple in the treatment of acute HRS for the last two decades in the U.S. Midodrine, an oral tablet, is like norepinephrine and produces vasoconstriction through alpha-1-adrenergic receptors.24 Octreotide injection is a somatostatin analogue that decreases the release of vasodilatory substances and glucagon leading to vasoconstriction of the splanchnic circulation.24 Because norepinephrine must be administered in the ICU, some healthcare facilities favor the combination of midodrine and octreotide. They also use midodrine/octreotide if they have not added terlipressin to their formularies.23 Unfortunately, patients tend to respond slowly to the combination and the combination requires an extended duration for full benefit.23 Octreotide cannot be given without midodrine but midodrine may be continued long-term (e.g., post-discharge) to maintain blood pressure in patients who are persistently hypotensive.23,29
Researchers recently published a single center experience with standardizing administration of midodrine and octreotide for treatment of HRS at their center.29 They wanted to standardize the use and dosing of albumin in combination with midodrine dosed at 2.5 to 10 mg three times daily and octreotide 50 to 100 mcg subcutaneously three times daily for 14 days and compare it to previous unstandardized prescribing. The goal was to obtain a full response: a serum creatinine within 0.3 mg/dL of baseline within seven to 14 days. Use of the standardized protocol was more effective in producing a full response than the historical unstandardized practice (25% vs 10%, p = 0.07).29 Additionally, the researchers also found that fewer patients in the protocol group required kidney replacement therapy. Guidelines suggest initiating midodrine at a dose of 7.5 mg three times daily and titrating it to 12.5 mg three times daily in combination with octreotide.23 The combination may still be in favor because it is a cost-effective alternative to terlipressin outside the ICU.
Midodrine is supplied in three tablet strengths which include 2.5 mg, 5 mg and 10 mg.30 This allows outpatient tapering or adjustment if the patient experiences tachycardia. Unfortunately, it is short acting and requires three times daily dosing initially. In practice, dropping the middle of the day dose without reducing the strength allows improved adherence once the patient’s blood pressure is stable. Midodrine’s labeling includes a boxed warning for possible marked elevation of supine blood pressure, and clinicians should monitor supine and standing blood pressure regularly.30
Octreotide is supplied in single dose ampules or multidose vials that must be stored in the refrigerator and protected from light; multidose vials must be discarded within 14 days. Octreotide is stable for 14 days at room temperature.31 Doses of 50 mcg to 100 mcg are administered every eight hours around the clock during the inpatient stay. If patients continue on octreotide as outpatients, the hospital pharmacy often needs to supply the doses. Patients and caregivers need appropriate education on subcutaneous injections and disposal of injection materials. In practice, the dose used in the hospital with success is often continued and not reduced to allow for the shortest duration possible. Octreotide subcutaneous injections on the outpatient side typically require additional insurance approval and preparation so discharge planning early is important.31
The Role of Pharmacists and Pharmacy Technicians in the Treatment of HRS
Pharmacists and pharmacy technicians can play an integral role in improving outcomes for patients presenting with or who have a history of HRS. Prevention is the key! Patients with end-stage liver disease should avoid medications that can precipitate HRS such as non-steroidal anti-inflammatory drugs and will require appropriate adjustment or discontinuation (if possible) of potential nephrotoxic agents (e.g., certain antimicrobials).
Ensuring patients with a history of spontaneous bacterial peritonitis (SBP) are on appropriate antibiotic prophylaxis can prevent subsequent SBP events that decrease blood flow to the kidneys. In the ambulatory setting, careful blood pressure monitoring and adjustment of blood pressure medications commonly used to treat portal hypertension (e.g., carvedilol), can ward off hypotensive events that can precipitate HRS.
Table 3 summarizes some lifestyle counseling tips that can help empower patients to play an active role in optimizing their care and preventing HRS episodes. Additionally, general management of concurrent disease states, such as heart failure and diabetes, can aid in maintaining optimal hemodynamics.
| Table 3. Lifestyle Counseling Points for Patients with Cirrhosis at risk for HRS35-37 | |
| Avoid alcohol | Even if the cause of liver damage isn’t drinking, alcohol use can increase the amount of damage. Patients who cease alcohol can experience dramatic improvements in some of the complications of cirrhosis. |
| Low sodium diet (especially in patients with ascites) | Limit sodium intake. This can be quite difficult, but if it can be done will help quite a bit with volume management. Patients with ascites are often asked to target ≤2 g/day. (For reference, 1 teaspoon of salt contains 2.3 g!) |
| Weight loss in patients who are overweight | Even a small amount of weight loss (e.g., a few pounds) can have a beneficial effect in patients with NAFLD or chronic HCV. |
| Protect yourself from infections | Patients need to stay up to date on vaccinations, wash their hands frequently, and avoid people who are sick. |
| Organize medication schedule | Patients with liver impairment can take seven to 10 medications a day—some administered multiple times a day. Investing in a strong adherence-enhancing system with alarm reminders or reminders from caregivers can be key. |
| Use OTC medications carefully | NSAIDs, such as ibuprofen and naproxen, can precipitate acute kidney injury. |
| Monitor weight daily (if on diuretic treatment) | Patients need to weigh themselves first thing in the morning after urinating. They should report significant weight changes to their providers (e.g., losing 1 pound or more a day or gaining more than 5 pounds in a week). |
SIDEBAR: Did you know…acetaminophen can be a great choice for patient with HRS?34
Imagine a situation where a medical intern is cross-covering in the medical intensive care unit and receives a call from a nurse about a patient with HRS. The patient is experiencing some mild pain and the nurse would like an as-needed medication to help.
Or…
You are working in the pharmacy and receive an order for oxycodone 5 mg every six hours as needed for mild pain. You are very concerned that this patient has both kidney and liver insufficiency and oxycodone is not a good choice but what else can you recommend?
What about acetaminophen?!?
Acetaminophen tends to have a bad rap mainly because it is in so many prescription and OTC products. It’s often in the news for causing liver toxicity. Oftentimes patients and providers do not think about the total acetaminophen exposure (the total daily dose of acetaminophen) and that is where the danger can come in. When the amount of acetaminophen’s toxic intermediary N-acetyl-p-benzoquinone imine (NAPQI) exceeds the liver’s glutathione stores, NAPQI starts to stick to hepatocytes (the liver’s main structural component). NAPQI acts like an antigen and stimulates the immune system to attack the liver.
Fortunately, it takes a significant amount administered at one time or consistently over several days to expend the glutathione stores. Doses up to 2,000 mg per day are safe and effective in most patients with severe liver insufficiency. (The maximum daily dose in healthy adults is 3900 mg.) Pharmacists and pharmacy technicians can ensure providers and patients with liver disease know they have alternative options. They can also help patients avoid reaching for a NSAID, especially if the patient has had a recent bout of HRS or if the patient is taking other medications that would suggest the presence of liver insufficiency (e.g., lactulose, rifaximin, norfloxacin). Remember prevention of HRS is the key!
When a hospital admits a patient, healthcare providers need to understand what the patient was taking at home and stop or continue the appropriate medications at the right doses. For example, prescribers should discontinue medications that could be reducing blood pressure (e.g., beta blockers and alpha beta blockers) on admission.11,16 They need to consider adjusting home medications for kidney dysfunction and restarting medications needed to manage other complications of end-stage liver disease (e.g., lactulose for encephalopathy).
When prompt administration of resuscitation with albumin is needed, the team may need help selecting the appropriate concentration. Patients who are significantly volume overloaded but have fluid in the extravascular space (e.g., in the abdominal cavity) would likely benefit from concentrated (25%) albumin. With the multidisciplinary team, the pharmacy team needs to understand the patient’s volume status and goals of therapy. Helping teams develop protocols to treat HRS can aid in goal-directed therapy and allow quick implementation of pharmacologic interventions to improve blood flow to the kidneys.
At discharge pharmacists and pharmacy technicians must ensure that medications are appropriately adjusted for the patient’s current kidney and liver function after the acute event has resolved or stabilized. The pharmacy team should be involved in educating patients on how to organize their new medication regimens, how to monitor their responses to therapy and recognize common adverse effects, and how appropriate lifestyle changes can increase the effectiveness of therapy and help avoid the advanced complications of liver disease.
CONCLUSION
HRS is a common complication for patients with advanced liver disease and ascites. Patients are in a state of decreased effective arterial blood flow to the kidneys and other end organs, and kidney injury is easily precipitated by nephrotoxic agents, over-diuresis, or bacterial infection. Acute treatment is aimed at restoring blood flow to the kidneys with the use of volume resuscitation and splanchnic vasoconstrictors. Pharmacists and pharmacy technicians can identify medications that may worsen kidney function, and assist in the appropriate prescribing, monitoring, and stewardship of these agents. Additionally, appropriate patient education—empowering patients to monitor their fluid/blood pressure status and avoiding OTC medications that can worsen their condition or precipitate HRS—is key in optimizing patient outcomes.
Pharmacist Post Test (for viewing only)
Set Your Ascites on Improving Patient Care: The Pharmacy Team’s Role in HRS Management
Pharmacist post-test
JC is a 56-year-old patient with end-stage liver disease secondary to non-alcoholic steatohepatitis (NASH) who presents to the emergency department with her caregiver after she was found disoriented in the backyard overnight. An arterial line is placed and the initial mean arterial pressure is 40 mmHg with a central venous pressure of 3 mmHg.
Past Medical History:
• Type 2 diabetes
• NAFLD, biopsy proven six years ago
• variceal bleed last year
• ascites and recent worsening encephalopathy.
Vital signs:
• blood pressure 72/30 mmHg
• temperature 102.3 F (39 C)
• weight 56 kg, last weight 58 kg one week ago
• no urine output
Labs:
• Scr 3.8 mg/dL (Scr 0.7 mg/dL last week).
No signs of edema or ascites.
Current medications: pantoprazole 40 mg daily, furosemide 40 mg every other day, carvedilol 6.25 mg twice daily, lactulose 30 mL TID, glipizide 10 mg daily, citalopram 10 mg daily.
Please use the case above to answer the next 5 questions.
1. JC’s blood pressure is 80/50 mmHg in triage, an arterial line is placed and CVP is initially 3 mmHg. What is the most appropriate immediate intervention given this information?
A. Normal saline 500 mL bolus
B. Vasopressin 0.04 units/min continuous infusion
C. Midodrine 10 mg three times daily
2. During JC’s admission the team requests your evaluation of the patient’s home medications. Which home medication would you discontinue on admission?
A. Carvedilol
B. Lactulose
C. Citalopram
3. What should the patient’s goal mean arterial pressure (MAP) be?
A. Increase MAP by 30%
B. Decrease MAP to 30 mmHg
C. MAP of at least 65 mmHg
4. The team is trying to determine what dose and concentration of albumin to administer. Based on only the information in the case, which initial dose and concentration is the most appropriate?
A. 100 grams of 5% albumin
B. 60 grams of 25% albumin
C. 60 grams of 5% albumin
5. The hospital is currently on ICU diversion and no critical care beds are available, so she must be cared for on the internal medicine unit. That unit cannot manage central lines. What is the most appropriate regimen to improve the patient’s MAP in addition to the currently infusing albumin?
A. Terlipressin
B. Norepineprhine
C. Octreotide
6. A patient has received terlipressin 1 mg every 6 hours for the past four days and the patient’s serum creatinine has increased from 3.5 mg/dL to 5 mg/dL. How should terlipressin be adjusted?
A. Stop terlipressin
B. Increase terlipressin dose to 1 mg every four hours
C. Increase terlipression dose to 2 mg every six hours
7. Which of the following medications should be avoided in patient with hepatorenal syndrome and/or liver cirrhosis?
A. Acetaminophen
B. Naproxen
C. Guaifenesin
8. HR is a 53-year-old Hispanic male who presents from hepatology clinic with an acute rise in serum creatinine. Admission medication reconciliation notes that his primary care doctor recently started him on losartan, and his blood pressure was 74/52 mmHg on admission. Following hydration with normal saline and stopping all other offending medications, the doctor prescribes midodrine and octreotide. What hemodynamic change can you expect following initiation of midodrine?
A. Decrease in blood pressure
B. Increase in portal pressure
C. Increased blood pressure
9. Which of the following is the most accurate rationale for combining albumin with other agents that cause vasoconstriction to manage hepatorenal syndrome?
A. Exogenous albumin administration decreases intravascular oncotic pressure and allows for a decrease in mean arterial pressure when combined with vasoconstricting agents
B. When specifically used in the setting of infection, exogenous albumin administration allows for enhanced delivery of protein bound antimicrobials to their required site of action
C. Use of intravenous concentrated albumin allows fluid from the extravascular space to be pulled into the blood stream and increases blood volume and delivery to the kidney
10. Which of the following best describes the pathophysiology of HRS?
A. Increased blood flow to the kidney in the setting of splenic vasodilation
B. Decreased blood flow to the kidney in the setting of portal hypertension
C. Decreased blood flow to the kidney in the setting of splenic vasoconstriction
11. Which of the following is a definitive treatment required to resolve HRS?
A. Liver transplant
B. Kidney transplant
C. Portal vein transplant
12. Which of the following best describes the main difference between Type I HRS and Type II HRS?
A. Type 1 HRS is associated with a higher rise in serum creatinine (at least 0.3 mg/dL from baseline).
B. Type 1 HRS happens more quickly (increase in serum creatinine over the most recent baseline taken within the past three months).
C. Type 1 HRS shows the presence of structural kidney disease (e.g., polycystic kidney disease or glomerular, interstitial, or vascular diseases).
Pharmacy Technician Post Test (for viewing only)
Set Your Ascites on Improving Patient Care: The Pharmacy Team’s Role in HRS Management
Pharmacy technician post-test
1. Which of the following is a reason that liver disease affects the kidneys?
A. Toxins that are cleared by the liver are toxic to the kidneys
B. The treatments for liver disease release nephrotoxins
C. Liver disease affects blood flow to the kidneys
2. Which of the following best describes main difference between Type I HRS and Type II HRS?
A. Type 1 HRS is associated with a higher rise in serum creatinine (at least 0.3 mg/dL from baseline) over any time period.
B. Type 1 HRS happens more quickly (increase in serum creatinine over most recent baseline taken within the past three months).
C. Type 1 HRS shows the presence of structural kidney disease (e.g., polycystic kidney disease or glomerular, interstitial, or vascular diseases).
3. A patient with a past medical history of cirrhosis and ascites comes into the pharmacy complaining of mild to moderate knee pain and asks for help picking an over-the-counter analgesic. Which of the following will the pharmacist most likely recommend because of safety concerns?
a. Naproxen
b. Low dose acetaminophen
c. Ibuprofen
4. A patient is picking up prescriptions for furosemide and spironolactone. Which of the following should the patient remember to do to prevent an over-diuresis that can precipitate HRS?
a. Weigh himself daily in the morning after he urinates; record his weights
b. Eat a high sodium diet; read labels carefully and aim for more than 2 grams/day
c. Practice good sleep hygiene; aim for an average 7 hours/night
5. JC is a 55-year-old patient admitted to the intensive care unit with worsening ascites and hepatorenal syndrome. His mean arterial pressure is 50 mmHg, and the ICU doctor orders IV crystalloids. Which home medication might the team want to discontinue?
A. Carvedilol
B. Lactulose
C. Citalopram
6. Which of the following is a definitive treatment required to resolve HRS?
A. Liver transplant
B. Kidney transplant
C. Portal vein transplant
7. Which of the following is a vasopressor of the splanchnic circulation?
A. Lactated ringers
B. Terlipressin
C. Albumin
8. Which of the following is the most accurate rationale for combining albumin with other agents that cause vasoconstriction in the management of hepatorenal syndrome?
A. Exogenous albumin administration decreases intravascular oncotic pressure and allows for a decrease in mean arterial pressure when combined with vasoconstricting medications
B. When specifically used in the setting of infection, albumin allows for enhanced delivery of protein bound antimicrobials to their required site of action
C. Use of intravenous concentrated albumin pulls fluid from the extravascular space into the blood stream and increases blood volume and delivery to the kidney
9. What is a drawback to the use of midodrine and octreotide in the treatment of HRS?
A. It constricts the splanchnic circulation.
B. It was not available in the United States until 2023.
C. It takes an extended number of days for full benefit.
10. Which of the following medications needs to be administered in the intensive care unit?
A. Albumin
B. Norepinephrine
C. Octreotide
References
Full List of References
References
1. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014 May 17;383(9930):1749-61.
2. Huang DQ, Terrault NA, Tacke F, Gluud LL, Arrese M, Bugianesi E, Loomba R. Global epidemiology of cirrhosis - aetiology, trends and predictions. Nat Rev Gastroenterol Hepatol. 2023 Jun;20(6):388-398.
3. Kim D, Alshuwaykh O, Dennis BB, Cholankeril G, Ahmed A. Trends in Etiology-based Mortality From Chronic Liver Disease Before and During COVID-19 Pandemic in the United States. Clin Gastroenterol Hepatol. 2022 Oct;20(10):2307-2316.e3.
4. Kondili LA, Buti M, Riveiro-Barciela M, Maticic M, Negro F, Berg T, Craxì A. Impact of the COVID-19 pandemic on hepatitis B and C elimination: An EASL survey. JHEP Rep. 2022 Sep;4(9):100531.
5. Blach S, Kondili LA, Aghemo A, Cai Z, Dugan E, Estes C, Gamkrelidze I, Ma S, Pawlotsky JM, Razavi-Shearer D, Razavi H, Waked I, Zeuzem S, Craxi A. Impact of COVID-19 on global HCV elimination efforts. J Hepatol. 2021 Jan;74(1):31-36.
6. Tariq R, Singal AK. Management of Hepatorenal Syndrome: A Review. J Clin Transl Hepatol. 2020 Jun 28;8(2):192-199.
7. Arroyo V. The liver and the kidney: mutual clearance or mixed intoxication. Contrib Nephrol. 2007;156:17-23.
8. Ginès A, Escorsell A, Ginès P, Saló J, Jiménez W, Inglada L, Navasa M, Clària J, Rimola A, Arroyo V, et al. Incidence, predictive factors, and prognosis of the hepatorenal syndrome in cirrhosis with ascites. Gastroenterology. 1993 Jul;105(1):229-36.
9. Biggins SW, Angeli P, Garcia-Tsao G, Ginès P, Ling SC, Nadim MK, Wong F, Kim WR. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021 Aug;74(2):1014-1048.
10. Wong F, Kwo P. Practical Management of HRS-AKI in the Era of Terlipressin: What the Gastroenterologist Needs to Know. Am J Gastroenterol 2023 Jun 1;118(6):915-920.
11. Chandna S, Zarate ER, Gallegos-Orozco JF. Management of Decompensated Cirrhosis and Associated Syndromes. Surg Clin North Am. 2022 Feb;102(1):117-137.
12. Patidar KR, Peng JL, Pike F, et al. Associations Between Mean Arterial Pressure and Poor ICU Outcomes in Critically Ill Patients With Cirrhosis: Is 65 The Sweet Spot? Crit Care Med. 2020 Sep;48(9):e753-e760.
13. Francoz C, Durand F, Kahn JA, Genyk YS, Nadim MK. Hepatorenal Syndrome. Clin J Am Soc Nephrol. 2019 May 7;14(5):774-781.
14. Maynard E. Decompensated Cirrhosis and Fluid Resuscitation. Surg Clin North Am. 2017 Dec;97(6):1419-1424.
15. Nanchal R, Subramanian R, Karvellas CJ, et al. Guidelines for the Management of Adult Acute and Acute-on-Chronic Liver Failure in the ICU: Cardiovascular, Endocrine, Hematologic, Pulmonary, and Renal Considerations. Crit Care Med. 2020 Mar;48(3):e173-e191.
16. Philips CA, Maiwall R, Sharma MK, et al. Comparison of 5% human albumin and normal saline for fluid resuscitation in sepsis induced hypotension among patients with cirrhosis (FRISC study): a randomized controlled trial. Hepatol Int. 2021 Aug;15(4):983-994.
17. Cullaro G, Kanduri SR, Velez JCQ. Acute Kidney Injury in Patients with Liver Disease. Clin J Am Soc Nephrol. 2022 Nov;17(11):1674-1684.
18. Mujtaba MA, Gamilla-Crudo AK, Merwat SN, Hussain SA, Kueht M, Karim A, Khattak MW, Rooney PJ, Jamil K. Terlipressin in combination with albumin as a therapy for hepatorenal syndrome in patients aged 65 years or older. Ann Hepatol. 2023, Vol. 28, p. 101126.
19. Kugelmas M, Loftus M, Owen EJ, Wadei H, Saab S. Expert perspectives for the pharmacist on facilitating and improving the use of albumin in cirrhosis. Am J Health Syst Pharm. 2023, Vol. epub.
20. Zheng X, Bai Z, Wang T, et al. Human Albumin Infusion for the Management of Liver Cirrhosis and Its Complications: An Overview of Major Findings from Meta-analyses. Adv Ther. 2023, Vol. 40, pp. 1494-1529.
21. Nassar Junior AP, Farias AQ, D' Albuquerque LA, Carrilho FJ, Malbouisson LM. Terlipressin versus norepinephrine in the treatment of hepatorenal syndrome: a systematic review and meta-analysis. PLoS One. 2014, Vol. 9, p. e107466.
22. Seshadri A, Appelbaum R, Carmichael SP 2nd, et al. Management of Decompensated Cirrhosis in the Surgical ICU: an American Association for the Surgery of Trauma Critical Care Committee Clinical Consensus Document. Trauma Surg Acute Care Open. 2022, Vol. 7, p. e000936.
23. Flamm SL, Wong F, Ahn J, Kamath PS. AGA Clinical Practice Update on the Evaluation and Management of Acute Kidney Injury in Patients With Cirrhosis: Expert Review. Clin Gastroenterol Hepatol. 2022, Vol. 20, pp. 2702-2716.
24. Flamm SL, Brown K, Wadei HM, Brown RS, Kugelmas M, et al. The Current Management of Hepatorenal Syndrome–Acute Kidney Injury in the United States and the Potential of Terlipressin. Liver Transplantation . 2021, Vol. 27, pp. 1191-1202.
25. Wong F, Curry MP, Reddy KR, Rubin RA, Porayko MK, Gonzalez SA, et al. Terlipressin plus albumin for the treatment of hepatorenal syndrome type 1. N Engl J Med 2021;384:818-828. N Engl J Med. 2021, Vol. 384, pp. 818-828.
26. Alessandria C, Ottobrelli A, Debernardi-Venon W, Todros L, Cerenzia MT, Martini S, Balzola F, Morgando A, Rizzetto M, Marzano A. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. J Hepatol. 2007, Vol. 47, pp. 499-505.
27. Martín-Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, Soriano G, Terra C, Fábrega E, Arroyo V, Rodés J, Ginès P and Investigators, TAHRS. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008, Vol. 134, pp. 1352-1359.
28. Terlivaz [package insert]. Mallinckrodt Pharmaceuticals;2023.
29. Hiruy A, Nelson J, Zori A, et al. Standardized approach of albumin, midodrine and octreotide on hepatorenal syndrome treatment response rate. Eur J Gastroenterol Hepatol. 2021, Vol. 33, pp. 102-106.
30. Midodrine [package insert]. Upsher-Smith Laboratories; 2020.
31. Octreotide Acetate Inejction [package insert]. Fresenius Kabi; 2022.
32. European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010 Sep;53(3):397-417.
33. Pitre T, Kiflen M, Helmeczi W, et al. The Comparative Effectiveness of Vasoactive Treatments for Hepatorenal Syndrome: A Systematic Review and Network Meta-Analysis. Crit Care Med. 2022 Oct 1;50(10):1419-1429.
34. Rogal SS, Hansen L, Patel A, Ufere NN, Verma M, Woodrell CD, Kanwal F. AASLD Practice Guidance: Palliative care and symptom-based management in decompensated cirrhosis. Hepatology. 2022 Sep;76(3):819-853.
35. Nobili V, Carter-Kent C, Feldstein AE. The role of lifestyle changes in the management of chronic liver disease. BMC Med. 2011 Jun 6;9:70.
36. Saleh ZM, Bloom PP, Grzyb K, Tapper EB. How Do Patients With Cirrhosis and Their Caregivers Learn About and Manage Their Health? A Review and Qualitative Study. Hepatol Commun. 2020 Nov 17;5(2):168-176.
37. US Department of Veterans Affairs. Ascites due to Cirrhosis. 2018. https://www.hepatitis.va.gov/pdf/ascites-fact-sheet.pdf Accessed 6/30/2023.