Learning Objectives
After completing this application-based continuing education activity, pharmacists will be able to
| · List the pathogenesis of MS |
| · Describe the first line and second line disease modifying therapies available for MS |
| · Personalize individualized treatment plans for the pregnant and pediatric MS patient |
| · Describe the pharmacist’s responsibilities for management of side effects associated with DMTs |
| · Explain vaccine recommendations for patients with MS |
After completing this application-based continuing education activity, pharmacy technicians will be able to
| · List MS’s primary signs and symptoms |
| · Point out warning signs of adverse reactions and nonadherence in patients taking DMTs for MS |
| · Identify patients for vaccinations |

Release Date:
Release Date: May 15, 2022
Expiration Date: May 15, 2025
Course Fee
FREE
GRANT INFORMATION
Educational Grant Funding for this CE is provided by:
Bristol Meyers Squibb
Novartis
ACPE UANs
Pharmacist: 0009-0000-22-039-H01-P
Pharmacy Technician: 0009-0000-22-039-H01-T
Session Codes
Pharmacist: 22YC39-KXV62
Pharmacy Technician: 22YC39-XEB48
Accreditation Hours
2.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-22-039-H01-P/T will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Bisni Narayanan, MS, PharmD
Pharmacy Supervisor- Operations
Yale New Haven Health Systems
Hamden, CT
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Dr. Narayanan has no relationship with ineligible companies and therefore have nothing to disclose.
ABSTRACT
Multiple Sclerosis (MS) is a chronic, inflammatory demyelinating and neurodegenerative disease of the central nervous system (CNS). Disease modifying therapies (DMTs) modify the disease’s natural course by reducing relapse and disability progression rates to improve patients’ quality of life. MS’s therapeutic landscape has changed vastly with the recent approval of several new DMTs. Similar to other autoimmune diseases, MS is more common in women than men. Women of childbearing age initiating DMTs need extensive counseling on safety during pregnancy and lactation. Pediatric MS is underdiagnosed and undertreated and coupled with exclusion of patients younger than 18 in clinical trials, therapy initiation can be complicated and delayed in pediatric patients. Pharmacists have a vital responsibility to monitor regular clinical follow up, surveillance of DMTs, and vaccine uptake in this population.
CONTENT
Content
Introduction
Until the mid-19th century, people who had the vague symptom constellation now recognized as multiple sclerosis (MS) were diagnosed with “paraplegias” (progressive neurological disorders with elements of motor impairment) or nervous disorders. Until then, the disease was usually diagnosed at autopsy, where “scleroses” (indurations or hardened areas) could be seen in the brain. French physician von Frerichs was the first to identify “scleroses” in living patients, and his colleague physicians Vulpian and Charcot teased MS from the other paraplegias as a separate neurologic entity with an episodic presentation.1 It was Charcot who drew the first macroscopic and microscopic images of MS lesions (see Figure 1), and the first patient he examined was one of his maids who had dysarthria (difficulty articulating words), ataxia (loss of the ability to control body movements), and tremor, now called Charcot's triad. (He initially thought she had syphilis.) Since then, researchers have developed a remarkable knowledge base about MS, but still, we have no cure.
FIGURE 1
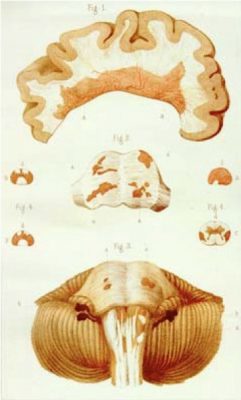
MS is a chronic, inflammatory, demyelinating, and neurodegenerative central nervous system (CNS) disease. In the United States, its projected prevalence in 2020 was 0.9 million cases and globally, the case count exceeded 2.8 million.2
MS in general is more prevalent in northern geographic latitudes, suggesting sunlight or vitamin D may influence its development. Although the life expectancy of people with MS has increased in the past 25 years, the median age of survival for people with MS is now 76 years compared to 83 years for the matched population. The International Advisory Committee on Clinical Trials of MS has defined four basic MS disease courses3:
- clinically isolated syndrome (CIS)
- relapsing- remitting (RRMS)
- secondary progressive (SPMS), and
- primary progressive disease (PPMS)
Clinicians and patients further describe this disease with the modifiers “activity” and “progression.” Disease activity refers to occurrence of a relapse or new activity on MRI, and progression refers to disability accrual independent of relapse activity during the progressive MS phase. Modifiers help accurately describe the patient’s current disease process.
Like the patients Frerichs and Charcot saw so long ago, MS’s initial symptoms may include Charcot’s triad (dysarthria, ataxia, and tremor), fatigue, visual impairment, motor weakness, reduced mobility, sensory loss, pain, impaired genitourinary function, depression, and cognitive impairment. MS is often labeled as a “silent” or “invisible” disease. MS’s presence or impact may not be immediately apparent to others because visual and sensory symptoms are not overtly visible. Silent disease progression can also occur with disability accrual in the absence of relapse activity.
Symptoms generally appear in adults between 20 and 50 years of age. RRMS is the most common disease course, affecting about 85% of patients who present with MS. It is characterized by an initial phase of relapses or exacerbations of neurologic episodes followed by periods of partial or complete recovery (remissions). In the secondary phase, neurologic disability accrues progressively.2
Returning to the days before MS had a name, physicians tried many nonspecific and dangerous remedies—among them deadly nightshade, arsenic, mercury, and injection of malarial parasites.
They tried many treatments that were considered useful for neurosyphilis. This continuing education activity focuses on today’s disease modifying therapies (DMTs). DMTs modify the disease’s natural course by reducing relapse and disability progression rates or inflammation to improve quality of life.
Since the approval of intramuscular interferon β-1a (IFNβ-1a) 25 years ago (the first DMT), MS’s therapeutic landscape has changed considerably. Clinicians face several challenges—selecting initial therapy, managing adverse effects, and reaching patients effectively in the pandemic era—while caring for patients with MS.4 MS treatment consumes significant healthcare resources, with treatment cost correlating closely with disease progression. According to a 2019 National MS Society analysis, MS’s estimated total economic burden in the US was $85.3 billion, with direct medical costs at $63.2 billion and indirect and nonmedical costs of $22.1 billion.5
Patients who have MS have multiple therapeutic options, from injections to oral therapy. Despite the convenience and autonomy offered by oral therapies, its use is often associated with poor adherence. About 1 in 10 patients with MS discontinued their first oral DMT within 6 months and 1 in 5 patients with MS did so within one year.6 Nonadherence increases MS-related hospitalization, relapse rates, morbidity, mortality, and healthcare costs. Infusion-based treatments administered by physicians have higher adherence rates. An important factor in adherence is many patients’ negative perception of lifelong therapy. Pharmacists can manage side effects, prevent drug interactions, and improve treatment adherence.
Pathogenesis
MS’s exact etiology and pathogenesis remain unclear.7 Researchers have proposed environmental, genetic, and infectious agents as factors influencing immune-mediated CNS injury. Both adaptive and innate immune responses play a role in MS pathogenesis.
- Antigen presentation to CD4+ lymphocytes prompts activation and proliferation of pro-inflammatory T lymphocytes (Th1 and Th17) leading to a downward cascade of pro-inflammatory cytokines.
- Up-regulation of adhesion molecules leads to T cell migration across the blood brain barrier in the CNS.
- The downstream inflammatory cascade of events includes B cell activation, proliferation and migration into the CNS, and recruitment of other inflammatory cells of CD8+ T cells, B cells, monocytes, macrophages, and microglia in the CNS.
These immune-mediated responses cause myelin, oligodendrocyte, and axonal damage—all of which occur in an acute inflammatory lesion. Axons that survive acute attacks may die from metabolic stress, and axonal loss correlates with disability in patients. Axonal damage can be identified by pathological changes and imaging studies.7
DISEASE MODIFYING THERAPIES
DMTs suppress and modulate the immune system to modify the disease’s course. DMTs act in the relapsing phase of the MS by exerting anti-inflammatory activity to reduce relapses and accumulation of MRI lesions and stabilize and delay disability. DMTs for MS can be grouped into infusion, oral, and injectable drugs.
PAUSE AND PONDER: If a recently-diagnosed patient with MS asked you about “first-line” therapies, what would you say?
The American Academy of Neurology practice guideline for DMTs in adults with MS recommends8
- Prescribing DMT to patients with a single clinical demyelinating event and two or more brain lesions characteristic of MS after discussing DMTs’ benefits and risks with patients
- Offering DMTs to patients with relapsing forms of MS with recent clinical relapses or MRI activity
- Prescribing alemtuzumab, fingolimod, or natalizumab for people with highly active MS
- Directing patients on DMTs to pharmaceutical support programs
- Switching DMTs when one of these factors occur: sub-optimal response to therapy, medication-related adverse effects, laboratory abnormalities, inadequate adherence, or when a more appropriate therapy is available
In many diseases, research has clearly defined first-, second-, and third-line approaches. For example, in hypertension, prescribers usually start with a diuretic and/or an ACE inhibitor. In cancer, oncologists turn to the National Comprehensive Cancer Network guidelines for specific types of cancer. Treatment is not so clear or structured in MS with one exception: alemtuzumab is never used first. It is used only if two other agents have failed to provide adequate response. Treatment must be tailored to the patient and is influenced by the patient’s presentation and symptoms, the disease’s progression or likelihood of progression, and the patient’s insurance coverage or lack thereof. Pharmacy technicians can be extremely helpful to patients who have financial barriers and help patients find financial assistance (see the SIBEBAR).
SIDEBAR: Financial Burden of MS Therapies51
The direct and indirect cost of managing MS is substantial. MS is the second most expensive chronic disease to manage, behind only heart failure. According to a recent study, among commercially insured patients with MS, the estimated direct healthcare cost exceeded $68,000 per year.
Drug therapies account for 70% of total healthcare expenditure in MS compared to national figures of 10% to 15% for other diseases. DMTs are the eighth most expensive therapeutic medication class—the national annual DMT spending in 2019 was $18.7 billion. Only 25% to 30% of people with MS are covered under the Medicare D program.
High and rising DMT prices translate to higher out of pocket copays, deductibles, and co-insurance for patients. This could lead to poor adherence, which in turn can lead to higher relapse rates, increased hospitalizations, and emergency room visits ultimately resulting in a vicious cycle of higher healthcare dollars. Pharmacy technicians can assist patients with MS in signing up for copay cards and applying for foundation assistance.
Since the Food and Drug Administration (FDA) approved the first MS drug almost two decades ago, experts have been unable to come to consensus on which treatment should be initiated first.9 Clinicians use two treatment approaches: escalation and induction therapy.
- The escalation approach emphasizes safety. If during prospective monitoring, a patient experiences breakthrough disease (e.g., relapses, MRI changes, or disability), then the prescriber switches the patient’s treatment to a higher efficacy agent. Clinicians employing this approach believe it spares patients potential side effects.
- The induction or highly effective early treatment approach rests on the theory that the ability to predict long-term MS outcomes and prospectively determine ongoing nervous system damage is limited. Hence, neurologists prescribe higher-efficacy agents early to alter the disease course, prevent irreversible disease progression, and minimize future disability.9
Among the currently approved agents (see Table 1), monoclonal antibodies (alemtuzumab, natalizumab, ocrelizumab, and ofatumumab) and cladribine have the highest efficacy. Sphingosine-1 phosphate receptor modulators and fumarates have intermediate efficacy and teriflunomide and platform therapies have the lowest efficacy.10
Table 1. Currently Approved Agents for MS10
| Drug Name (Brand) |
Recommended Dose |
| Fumarates | |
| Dimethyl fumarate (Tecfidera) | 120 mg PO BID for 1 week, then 240 mg PO BID |
| Diroximel fumarate (Vumerity) | 231 mg PO BID for 7 days, then 462 mg PO BID |
| Monomethyl fumarate (Bafiertam) | 95 mg PO BID for 7 days, then 190 mg PO BID |
| Monoclonal Antibodies | |
| Alemtuzumab (Lemtrada) | Year 1: 12 mg/day IV infusion on 5 consecutive days
Year 2: 12 mg/day IV infusion for 3 consecutive days. Year 3&4- no treatment |
| Natalizumab (Tysabri) | 300 mg IV infusion every 4 weeks |
| Ocrelizumab (Ocrevus) | Start dose: 300 mg IV infusion, followed by 300 mg IV infusion 2 weeks later
Subsequent dose: 600 mg IV every 6 months |
| Ofatumumab (Kesimpta) | Week 0,1 &2: 20 mg SC
Week 4: 20 mg per month starting at week 4 |
| Platform therapies | |
| Glatiramer acetate (Copaxone, Glatopa) | 20 mg SC QD
40 mg SC TIW |
| Interferon beta-1b (Avonex) | 30 mcg IM once weekly |
| Interferon beta-1b (Betaseron, Extavia) | 250 mcg SC every other day |
| Interferon beta-1b (Rebif) | 22-44 mcg SC TIW |
| PegINF beta-1b (Plegridy) | 125 mcg SC or IM every 14 days |
| Purine analogue | |
| Teriflunomide (Aubagio) | 7 mg or 14 mg PO QD |
| Pyrimidine synthesis inhibitor | |
| Cladribine (Mavenclad) | 3.5 mg/kg of body weight divided into two yearly treatment courses. Each treatment course divided is divided into two treatment cycles of 4-5 days separated by 4 weeks |
| Sphingosine-1 phosphate receptor modulators | |
| Fingolimod (Gilenya) | 0.5 mg PO QD |
| Ozanimod (Zeposia) | 0.23 mg PO QD on days 1-4, 0.46 mg PO QD on days 5-7, 0.92 mg PO QD on day 8 and thereafter |
| Ponesimod (Ponvory) | 2 mg PO QD, titrated over 15 days to maintenance dose of 20 mg PO QD |
| Siponimod (Mayzent) | 0.25 mg PO QD, titrated to 2 mg PO QD over a six-day period |
ABBREVIATIONS: BID = twice daily; IM = intramuscular; IV = intravenous; PO = oral; QD = once daily; SC = subcutaneous; TIW = three times a week
PAUSE AND PONDER: Can you list the generic names of the monoclonal antibodies used in MS? Can you think of good reasons to know the generic and brand names?
Platform Therapies
The first DMTs approved for MS were IFNβ-1a and glatiramer acetate, both injection therapies.11 Platform therapies were the mainstay of therapy until the FDA approved some oral agents. In patients with relapsing MS, the initial phase 3 trial of IFNβ-1a showed this biologic reduced relapse rates by 18% to 34% compared to placebo.11 These agents have more long-term safety data available than the newer agents. Common adverse effects include injection site reactions, myalgias, and flu-like symptoms. Glatiramer acetate can cause lipoatrophy, vasodilation, and rash in addition to injection site reactions.10
Monoclonal Antibodies
Many current therapies are monoclonal antibodies, all of which end in the suffix -mab.
Anti-CD20 Therapies
CD20 is a transmembrane ion channel protein expressed on the surface of B cells. Anti-CD20 therapies deplete circulating CD20+ B-cells through mechanisms of antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDCC), and antibody-triggered apoptosis (programmed cell death).12 Anti-CD20 treatment reduces the proliferation of pro-inflammatory cytokines CD4+ and CD8+ T cells while increasing regulatory T cells. Researchers have studied four anti-CD20 monoclonal antibodies in MS: ocrelizumab, ofatumumab, rituximab, and ublituximab. They differ in structure, binding site, immunogenicity, degree of ADCC, and CDCC.12 Ublituximab is an investigational anti-CD20 monoclonal antibody and rituximab is commonly prescribed off-label MS treatment and will not be discussed. All monoclonal antibodies are infusion therapies (with the exception of ofatumumab which is available as prefilled syringes and autoinjector pens for subcutaneous use) with a less frequent dosing frequency compared to platform therapies.
Ocrelizumab
Ocrelizumab is intravenously administered and selectively targets and binds to the CD20 epitope on B-cells to trigger ADCC.13 Ocrelizumab is approved for the treatment of PPMS, relapsing forms of MS including CIS, RRMS, and active SPMS. Phase 3 randomized controlled trials (OPERA I and OPERA II) compared ocrelizumab’s efficacy (600 mg IV every 24 weeks) with subcutaneous IFNβ-1a (44 mcg three times weekly) for 96 weeks in patients with RRMS. In both studies, the annualized relapse rate (ARR) in ocrelizumab-treated patients decreased significantly compared to IFNβ-1a at 96 weeks. The proportion of ocrelizumab-treated patients had less confirmed disability progression than IFNβ-1a-treated patients at 12 and 24 weeks. The open-label extension phase showed that early initiation and continuous ocrelizumab treatment for up to five years was associated with a reduction of confirmed disability progression compared to switching to ocrelizumab after two years of IFNβ-1a. Patients with PPMS receiving ocrelizumab had lower 12- and 24- week confirmed disability progression compared to placebo. Post hoc analysis of the open-label extension of the phase 3 ONTARIO trial showed ocrelizumab was associated with sustained benefits on measures of disease progression over 6.5 years of follow up.13
Common adverse reactions with ocrelizumab infusion include respiratory and herpes infections and infusion reactions. Clinical staff must observe patients for at least one hour post infusion.13
Ofatumumab
Ofatumumab is a humanized, IgG1 monoclonal antibody that binds to a distinct site from ocrelizumab.14 Ofatumumab exhibits marked CDCC activity with decreased ADCC. The MIRROR trial assessed the dose-response of subcutaneous ofatumumab on efficacy and safety outcomes in relapsing forms of MS. The researchers randomized patients with RRMS to subcutaneous ofatumumab 3, 30, or 60 mg every 12 weeks, subcutaneous ofatumumab 60 mg every 4 weeks for 24 weeks, or placebo followed by subcutaneous ofatumumab 3 mg at week 12. Imaging studies showed all patients in all ofatumumab groups had 65% fewer cumulative new lesions than patients who received placebo. Dose dependent B-cell depletion was observed, and complete depletion was unnecessary for robust treatment effect.14 In the phase 3 ASCLEPIOS I and II trials, subcutaneous ofatumumab was associated with lower ARR compared to teriflunomide, an oral pyrimidine synthesis inhibitor.15 Despite the advantage of subcutaneous administration, clinicians must monitor patient adherence as closely as they monitor adherence to IV administration.
Adverse reactions include injection site reactions, upper respiratory tract infections, and headache.
Anti-Integrin Therapy
Natalizumab prevents binding of integrins at their endothelial receptors, blocking lymphocyte entry into the CNS.16 In the AFFIRM study, patients with relapsing MS received natalizumab (300 mg IV every four weeks) or placebo.16 At the one-year mark, patients in the treatment arm showed a 68% reduction in ARR compared to placebo.
Adverse reactions include headache, fatigue, urinary tract infection, urticaria, vaginitis, depression, and diarrhea. Of note, in February 2005, the manufacturer voluntarily temporarily withdrew the drug following the death of two patients from progressive multifocal leukoencephalopathy (PML) (See SIDEBAR). Because of the risk of PML, natalizumab is now only available through a restricted REMS program called the TOUCH Prescribing Program.16 Only prescribers, pharmacies, and infusion sites enrolled in the program can prescribe, distribute, or infuse natalizumab. Patients must acknowledge the risks of treatment including PML and opportunistic infections by signing the Patient-Prescriber Enrollment Form.
SIDEBAR: What is Progressive Multifocal Leukoencephalopathy?50
Progressive multifocal leukoencephalopathy (PML) is a rare infection caused by the John Cunningham virus (JCV). The virus attacks and damages the myelin sheath (the material that insulates and protects the nerve cells) leading to demyelination. About 85% of the general adult population carries the JCV virus, but it is inactive in healthy individuals.
In immunocompromised patients JCV reactivation can lead to deadly consequences. Clinical manifestations of PML can vary. Most patients experience ataxia, gait disturbance, and visual and cognitive dysfunction. There is no specific treatment for PML. The current recommendation for PML is remove the offending agent along with supportive care. Natalizumab is commonly associated with PML, followed by fingolimod, and other DMTs including dimethyl fumarate, ocrelizumab and alemtuzumab. A combination of clinical and imaging findings along with the presence of cerebrospinal JCV by PCR is used for diagnosing PML.
Anti-CD52
Alemtuzumab is directed towards the CD52 surface antigen on B- and T-lymphocytes, natural killer cells, monocytes, and macrophages.17 Alemtuzumab is indicated for the treatment of relapsing forms of MS. It is usually reserved for patients with inadequate responses to two or more previous MS drugs.
In the CARE-MS I study, researchers randomized patients with untreated RRMS to alemtuzumab 12 mg/day intravenous (five consecutive days; 12 months later: three consecutive days) or IFNβ-1a 44 mcg subcutaneous three times a week.18 Alemtuzumab-treated patients experienced an ARR reduction of 54.9% compared to those treated with IFNβ-1a. The CARE MS II trial compared the efficacy and adverse events of alemtuzumab and IFNβ-1a in patients with RRMS who had failed first-line therapies.19 Alemtuzumab reduced the relapse rate by 49.4% compared to IFNβ-1a.
Alemtuzumab’s adverse reactions include autoimmune diseases and infusion reactions. Clinicians must monitor patients for two hours after each infusion. Patients should receive acyclovir prophylaxis during the first two months post infusion or until CD4 lymphocytes are less than 200 cells/mcL. Patients usually need acyclovir prophylaxis for the first 24 months, as about 80% of patients may not reach CD4 counts less than 200 cells/mcL until 12 months after infusion.17
Alemtuzumab is a restricted distribution drug and is available only via a REMS program due to risk of autoimmunity, infusion reactions, and malignancies.
Oral Therapies
Fumarates
Fumarates are esters or salts of fumaric acid, a compound that is found in nature, has a fruity taste, and is sometimes used as a food additive. It’s an intermediary in the cellular energy-producing cycle (Kreb’s cycle) and a product of the urea cycle. The fumarates’ exact mechanism of action in MS is unknown. Fumarates may activate the nuclear factor (erythroid-derived 2)–like 2 (Nrf2) pathway for anti-inflammatory and cytoprotective effects.20 Dimethyl fumarate, diroximel fumarate, and monomethyl fumarate are oral medications indicated for the treatment of relapsing forms of MS including CIS, RRMS, and active SPMS. Dimethyl fumarate’s common adverse effects include flushing, diarrhea, nausea, and abdominal pain. Patients generally tolerate treatment well but rare cases of PML have been recorded.
Diroximel fumarate is a second-generation fumarate, approved in 2019 based on the bioequivalence, safety, and efficacy data for dimethyl fumarate.20 Both dimethyl fumarate and diroximel fumarate are converted into monomethyl fumarate, the pharmacologically active metabolite. In the EVOLVE-MS-2 phase 3 trial, diroximel-treated patients (462 mg PO twice a day) experienced fewer gastrointestinal (GI) side effects and lower discontinuation rates due to GI side effects compared to dimethyl fumarate (240 mg PO twice a day).21
The FDA approved monomethyl fumarate in April 2020 for the treatment of relapsing forms of MS. In a randomized, five-week study, patients on monomethyl fumarate (190 mg PO twice a day) showed an improved GI tolerability profile compared to dimethyl fumarate treatment arm (240 mg PO twice a day).22 A distinct feature of fumarate therapy is the gender distribution of GI adverse effects. Females are more likely to experience treatment-related GI adverse effects compared to males.22
Teriflunomide
Teriflunomide is a once daily oral immunomodulator approved in 2012 for the treatment of RMS, including CIS, RRMS and active SPMS.23 It reversibly inhibits dihydroorotate dehydrogenase, a mitochondrial enzyme required for the de novo pyrimidine synthesis. Inhibition of de novo pyrimidine synthesis reduces the number of activated T and B cells available to enter the CNS. Its common adverse effects are headache, diarrhea, nausea, hair thinning, and elevated alanine aminotransferase levels (ALT).
Since teriflunomide is an active metabolite of leflunomide, it is contraindicated in patients on current leflunomide treatment. Leflunomide is used alone or in combination with other medications to treat rheumatoid arthritis. The TEMSO and TOWER trials demonstrated teriflunomide’s clinical effectiveness. Compared to placebo, it significantly reduced relapse rates and disability progression at the dose of 14 mg daily in adults. Results from clinical trials with direct comparisons to other DMTs vary. Ofatumumab was associated with lower ARR and lower rates of disability worsening compared to teriflunomide, but similar rates of disability improvement and brain volume loss. Ponesimod (discussed in next section) was associated with lower ARR and improvement in fatigue compared with teriflunomide.23
Sphingosine-1 Phosphate Receptor Modulators
Sphingosine-1 phosphate receptors (S1PR) are expressed in multiple organs and systems.24 The S1PR axis is implicated in several immune mediated disorders. Lymphocyte cell surfaces express sphingosine-1 phosphate receptor subtype 1 (S1PR1); S1PR modulators bind to the receptor, sequestering lymphocytes within the lymph nodes and preventing their migration to the CNS.24
Currently, the FDA has approved four S1PR1 modulators to treat MS: fingolimod, siponimod, ozanimod, and ponesimod.24 Fingolimod has a broad affinity for all S1PR subtypes, and the rest were developed with greater selectivity for the S1PR1 subtype. The second generation S1PR1 modulators have shorter half-lives, which allows rapid reversal of pharmacological effects upon discontinuation. This is beneficial when managing treatment-related complications, serious or opportunistic infections, and drug elimination during pregnancy.25
The first dose of fingolimod acts as an agonist activating S1PR1 leading to bradycardia and heart block. Subsequent doses downregulate S1PR1 and cardiac effects resolve.26 Patients initiating fingolimod require first dose observation for at least six hours with electrocardiogram before and at the end of the observation period and pulse and blood pressure monitoring hourly. First dose observation is not required for siponimod and ozanimod in the absence of cardiac history, as patients gradually increase the dose over several days.26
Vascular effects of S1PR modulators can produce other adverse effects.24 Prior to S1PR modulator treatment initiation, the manufacturer recommends an ophthalmologic assessment, especially for patients with a history of diabetes, macular edema, or uveitis due to risk of macular edema. CYP2C9 genetic testing is required prior to siponimod treatment initiation to determine the titration and dosing schedule. Siponimod is metabolized through the cytochrome P450 system primarily through CYP2C9 enzyme. Heterozygote individuals (who have a different version of gene from each parent) with the *3 variant of CYP2C9 enzyme exhibit slower siponimod metabolism.46 However, homozygous individuals CYP2C9 *3/*3 (same version of the gene from each parent) will have severely impaired drug metabolism. Heterozygotes will need lower dose titration and lower final dose. Siponimod is contraindicated in CYP2C9 *3/*3 genotype.
One death occurred in a phase 3 trial of fingolimod due to disseminated varicella infection. For this reason, patients starting S1PR modulators must undergo testing for varicella zoster virus (VZV) antibodies. If they have no antibodies, the recommendation is to delay treatment until VZV vaccination is complete.24
Cladribine
Cladribine is a synthetic purine nucleoside analogue that selectively depletes peripheral lymphocytes.27 It was initially developed to treat hematological malignancies. Cladribine is the first short-course DMT approved for MS. Patients take two short courses one year apart followed by 2 years of no treatment. Patients are monitored during the 2-year treatment course and for at least another 2 years of no treatment. The safety and effectiveness of restarting cladribine more than 2 years after completing therapy is unknown. Analysis from CLARITY extension study shows benefits of cladribine tablets sustained for 6 years.49
Common adverse effects are upper respiratory tract infections, headache, and lymphopenia. Patients must be screened to exclude malignancies, infections, and pregnancy prior to initial treatment. Clinicians need to obtain a baseline MRI due to risk of PML.27
DMTs in Special Populations
MS affects women of childbearing age (20 to 40 years) disproportionately and is three times more common in women than men.4 Up to 25% of women have reported planning a pregnancy within two years of diagnosis. About 30% of women with MS will deliver a child after disease onset. Female patients have higher relapse rates and inflammatory lesions than men, but male patients reach SPMS sooner than women.4 People with MS are encouraged to start treatment early to prevent long-term disability accrual. About 20 years ago, the main recommendation was to abandon treatment during pregnancy. With available treatment options, DMT is the first line treatment option for women with MS, due to its effectiveness in lowering MS severity, relapses, and progression of CNS lesions.
Preconception Counseling
Women of childbearing age with MS (WMS) need preconception counseling.24 Clinicians must consider various drugs’ half-lives and wash out periods, and potential for disease reactivation if they discontinue drugs in WMS contemplating a future pregnancy (see Figures 2 and 3).4
Figure 2. Safety and Efficacy of DMTs in Pregnancy28
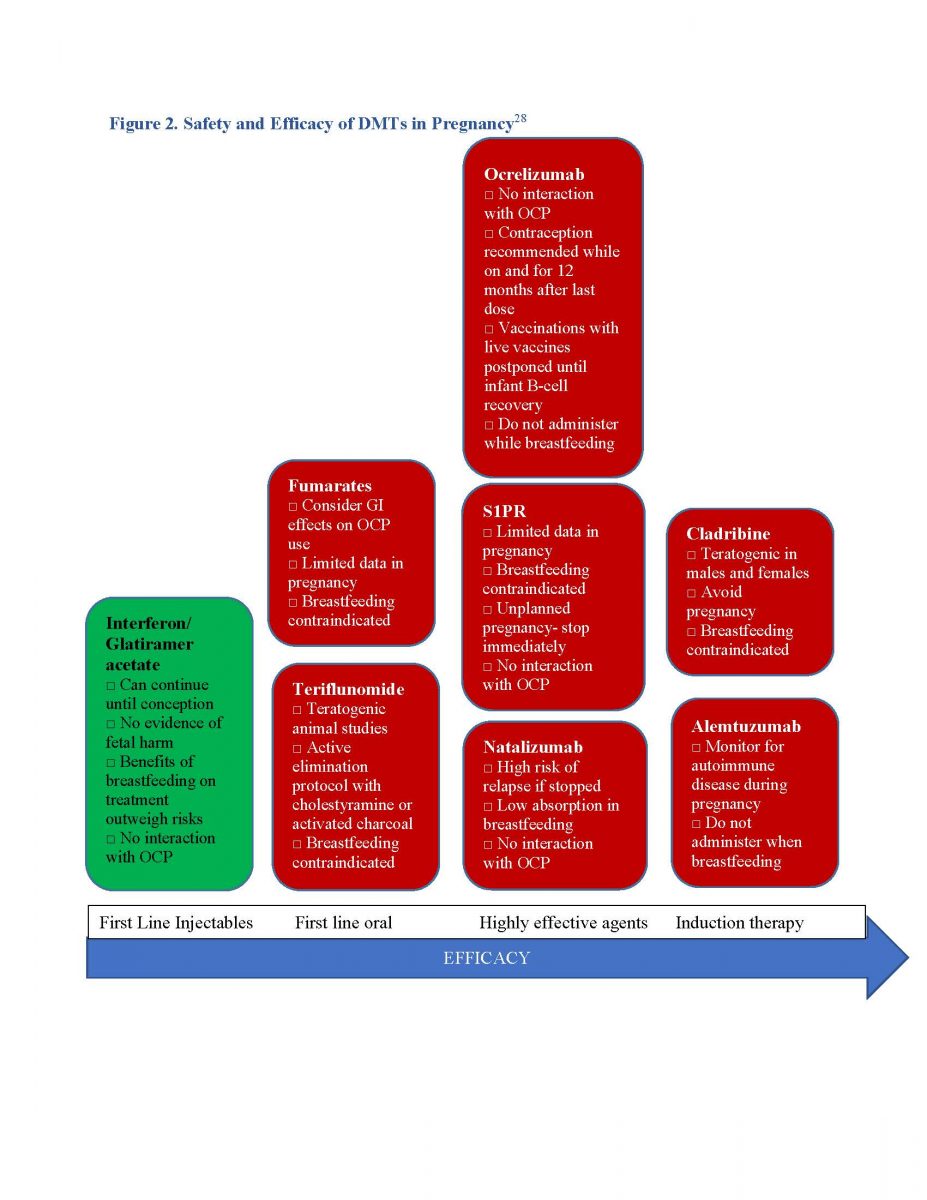
Figure 3. Recommended DMT wash out period for pregnancy28
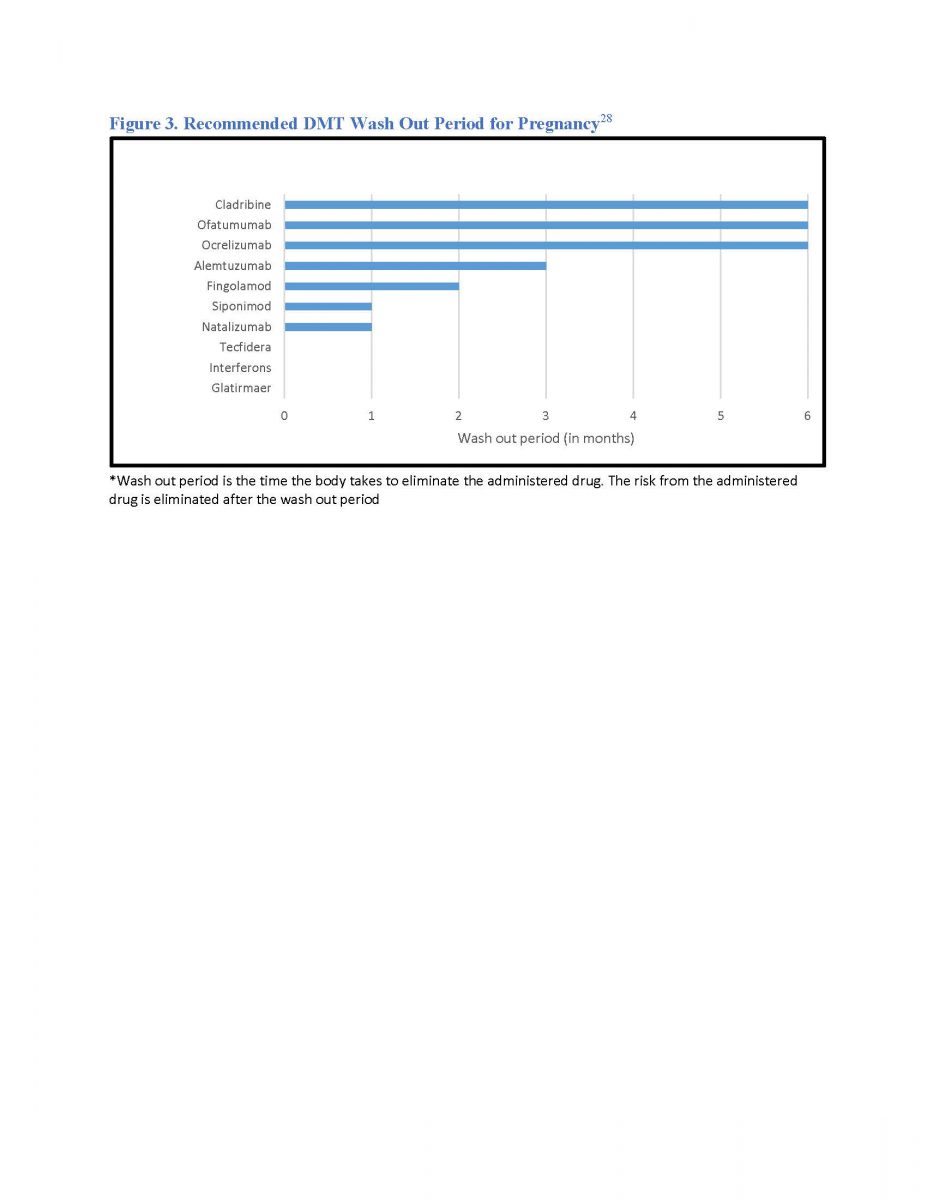
*Wash out period is the time the body takes to eliminate the administered drug. The risk from the administered drug is eliminated after the wash out period
The landmark Pregnancy in Multiple Sclerosis (PRIMS) study examined the effect of pregnancy and postpartum state on MS’s course along with breastfeeding and epidural analgesia.28 The study demonstrated that relapse rates decline in WMS during the third trimester but increase within three to four months postpartum, especially for patients with active preconception MS. For some women the best choice is to achieve disease control/stability with highly effective DMTs prior to conception. For WMS already on DMT, prescribers need to discuss any switch or wash out period in advance to minimize relapse risk and fetal exposure. The overall birth rate is slightly lower in WMS than the general population. This could be attributed to the birth choices by WMS. Clinicians should reassure WMS that the majority of pregnancies result in a healthy baby.28
PAUSE AND PONDER: What are the common adverse effects of DMTs for multiple sclerosis?
Pregnancy and Post-Partum
In women with relatively mild MS, discontinuing medication during pregnancy is an acceptable risk.28 An appropriate strategy for women with more active disease is to continue the medication during pregnancy, while mitigating risk to the fetus. First-line injectable medications (IFNβ-1a and glatiramer acetate) are generally considered safe during pregnancy as these are large molecules and do not cross the placenta. If women stop medications and restart them post-partum, it may take three months to reach full efficacy. Oral DMTs are small molecules and cross the placenta.28
Monoclonal Antibodies
The transplacental transfer of IgG in the first trimester is negligible.28 Hence, monoclonal antibodies can be dosed close to conception. In the second or third trimester, the monoclonal antibody will cross the placenta, with active transport in the third trimester increasing fetal exposure.
Natalizumab
Natalizumab’s biologic duration is short with dosing every four to eight weeks to prevent disease reactivation.28 Discontinuing natalizumab before or during pregnancy can cause disease reactivation and accumulation of permanent irreversible disability. Natalizumab exposure in pregnancy is associated with hematological abnormalities, low birth weight, and increased hospitalizations in the baby’s first year of life. Women with highly active MS receiving natalizumab may switch to an alternative high efficacy agent (ocrelizumab or alemtuzumab) to prevent rebound relapses. Another option is to extend the dosing interval to six to eight weeks during pregnancy with last dose no later than 34 weeks of gestation. Natalizumab should be re-dosed promptly post-partum.28
Anti-CD20 Therapies
Ocrelizumab’s biannual dosing schedule is conducive to attempting a pregnancy with minimal drug exposure.28 Ocrelizumab is eliminated roughly four months after infusion. Since IgG does not cross the placenta in the first trimester, women with more active MS can attempt to become pregnant one to three months after infusion. If women are unsuccessful by nine months after dosing, another dose can be administered followed by attempts at conception. Use of anti-CD20 antibodies during pregnancy should be reserved for women with aggressive MS due to limited data.28
Alemtuzumab
Alemtuzumab efficacy on inflammatory MS activity has been demonstrated to extend for more than 12 years following initial treatment.29 Conception can occur four months following treatment with subsequent doses if required delayed to sometime after pregnancy. Clinicians should stress the importance of monitoring thyroid function and complete blood count due to potential risk of unrecognized thyroid disease or thrombocytopenia to both mother and child.28
Treatment of Relapses during Pregnancy and Postpartum
The first-line treatment option for relapses during pregnancy or breast feeding is short courses of high-dose methylprednisolone.28 Studies have shown a slight increased risk of adverse fetal outcomes including cleft palate and low birth weight from corticosteroid exposure. Prescribers should reserve corticosteroids for clinically significant relapses and avoid first-trimester exposure. Severe or refractory relapses can be treated with plasma exchange with associated risks of thromboembolic events.28
Pediatric Multiple Sclerosis
Pediatric multiple sclerosis (PMS; MS occurring in children younger than 18 years of age) accounts for 3% to 5% of the MS population.30 PMS has distinctive features with a differing course than that seen in adults. About 98% of PMS presents with RRMS; readers will recall that RRMS accounts for 85% of MS in adults. Children with PMS accumulate more MRI lesions, reach disability milestones at earlier ages, and have more long-term cognitive impairment than adults. Children also exhibit higher relapse rates than adults. However, after the initial period of the disease, children’s high CNS plasticity and regeneration of brain damage usually leads to complete symptom remission. Common symptoms reported in children with PMS include sensory, motor, and brainstem dysfunction. Pediatric and adult-onset diseases have common genetic and environmental risks.30
PAUSE AND PONDER: How does treatment of pediatric and adult MS differ?
Treatment
Traditionally, the first-line treatment in children with PMS is IFNβ-1a or glatiramer acetate due to their favorable safety profiles.31 Researchers studied IFNβ-1a’s safety and tolerability in a cohort of 43 children with mean age of 13 years and eight children younger than 10. The most common adverse effects included flu-like symptoms, abnormal liver function tests, and injection site reactions. Another large retrospective study, REPLAY, examined interferon-beta’s safety and efficacy in children, reviewing records of children aged 2 to 17. The ARR was 1.79 before treatment, and 0.47 during treatment.32
Fingolimod is the only FDA-approved DMT for affected individuals younger than 18.33 In a randomized, double-blind trial, researchers compared fingolimod to intramuscular IFNβ-1a. They assigned patients aged 10 to 17 with RRMS to receive fingolimod 0.5 mg orally per day (0.25 mg per day for body weight 40 kg or less) or intramuscular IFNβ-1a 30 mcg per week for up to two years. The ARR was 0.12 with fingolimod compared with 0.67 with IFNβ-1a. MS relapses occurred in 88.8% of fingolimod patients versus 95.3% of IFNβ patients. Over two years, fingolimod was associated with lower relapse rates and less accumulation of lesions on MRI than IFNβ. However, the fingolimod-treated population had higher rates of adverse events (infection, leukopenia, and convulsions).33
The majority of adult and pediatric patients have a relapsing-remitting course. Hypothetically, treatments proven effective in adults could theoretically be used in children. However, recruiting pediatric patients for clinical trials has several challenges.34 Given the low prevalence of PMS, recruitment into a clinical trial is a prolonged process. A consensus statement from the International Pediatric Multiple Sclerosis Study group mentions placebo-controlled trials of immunomodulatory agents proven effective in adult MS are inappropriate in the pediatric MS population. This reflects the ethical dilemma of enrolling children into a placebo group given the high relapse rate and more rapid accrual of new lesions in the pediatric population relative to the adult-onset disease.34
Vaccine Recommendations
Clinicians should assess infectious disease history and review previous immunity to vaccine-preventable infections at the time of MS diagnosis. They should offer vaccinations as soon as possible to prevent treatment delays caused by need to complete the vaccination series. Patients with MS experiencing a relapse should delay vaccination until resolution or symptom improvement. In patients on immunosuppressive treatments, attenuated vaccines are contraindicated due to the risk of developing vaccine-transmitted disease (see Table 2).35 DMTs may also modulate the effectiveness of inactivated vaccine by lowering the immune system’s capacity to mount an effective response. However, patients with MS should receive the seasonal influenza vaccine to reduce vaccine-preventable hospitalization and mortality.35
Table 2. Live Vaccine Considerations for Patients with MS on DMTs27,39-47
| Drug name | Special considerations |
| Alemtuzumab | Complete necessary immunizations at least 6 weeks prior to treatment. Prior to treatment, check for varicella history or vaccination. If antibody negative, consider vaccination |
| Cladribine | Complete live or live attenuated vaccines within 4-6 weeks preceding treatment |
| Fingolimod | Avoid the use of live attenuated vaccines during and for 2 months after treatment because of the risk of infection |
| Fumarates, platform therapies, natalizumab | No special considerations |
| Ocrelizumab | Complete live or live-attenuated vaccinations at least 4 weeks and non-live vaccinations at least 2 weeks prior to initiation |
| Ofatumumab | Complete live or live-attenuated vaccinations at least 4 weeks and non-live vaccinations at least 2 weeks prior to initiation |
| Ozanimod | Complete live vaccinations at least 1 month prior to treatment initiation. Avoid live vaccines during and for 3 months after treatment cessation. Prior to treatment, check for varicella history or vaccination. If antibody negative complete VZV vaccination, postpone ozanimod treatment for 4 weeks. |
| Ponesimod | Avoid live attenuated vaccines during and for up to 1-2 weeks after treatment. Complete live attenuated vaccine at least 1 month prior to initiation. If antibody negative complete VZV vaccination, postpone ponesimod treatment for 4 weeks |
| Spinomod | Avoid live attenuated vaccines during and for 4 weeks after treatment cessation. Discontinue treatment 1 week prior and until 4 weeks after a planned vaccination. Prior to treatment check for varicella history or vaccination. If antibody negative complete VZV vaccination, postpone spinomod treatment for 4 weeks |
A number of immune mediated processes are involved in mounting a defense against infection after a vaccination. Vaccination leads to a downward cascade of immune reactions, with the final step involving B-cell transformation into plasma cells and production of antigen-specific antibodies. B-cell depleting therapies are a major concern due to their direct impact on humoral response. Researchers studied the effect of B-cell depleting therapy, ocrelizumab, on vaccine responses in patients with MS.36 In patients receiving ocrelizumab, seroconversion frequency and antibody titer was reduced after the 23-valent pneumococcal polysaccharide vaccine (23-PPV). Boosting with the 13-valent conjugate pneumococcal vaccine (13-PCV) four weeks after the first dose did not enhance response to pneumococcal serotypes 13-PCV has in common with 23-PPV. Despite the blunted vaccine response with the anti-CD20 antibodies, vaccinations can be expected to be protective.36
With the ongoing COVID-19 pandemic, appropriate immune response against the SARS-CoV-2 virus is vital especially in patients with MS receiving DMTs (see Table 3). In a recent study, patients with MS demonstrated protective response six months following PfizerBNT162b2 vaccination.37 Positive humoral IgG response was demonstrated in 9.5% of fingolimod treated patients, 22.8% of ocrelizumab treated patients, and 86.4% of alemtuzumab treated patients. All patients treated with cladribine, dimethyl fumarate, natalizumab, and teriflunomide exhibited positive humoral response which was comparable to untreated patients with MS. The Centers for Disease Control now recommends a third booster dose for the COVID-19 vaccination series. The third COVID-19 booster dose is safe in patients with MS, with no increased risk of relapse activity.38
Table 3. MS Society Guidelines for COVID-19 Vaccine Series48
| Drug Class/Drug Name | Guidance |
| Alemtuzumab | Receive vaccine 4 weeks prior to treatment initiation. If patient is already on the infusion, vaccinate 24 weeks or more after the last dose. If patient is due for next dose, resume infusion 4 weeks or more after full vaccination of 2 doses. |
| Beta interferons, glatiramer acetate, fumarates, natalizumab, teriflunomide | Can receive vaccine, no DMT adjustment required |
| Cladribine | Receive vaccine 2 weeks prior to treatment initiation. Limited data exists for vaccination while on cladribine. |
| Ocrelizumab | Receive vaccine 2 weeks prior to infusion. If patient is already on infusions, can complete vaccination 12 weeks or more after last ocrelizumab dose. Resume 4 weeks or more after getting fully vaccinated |
| Ofatumumab | Receive vaccine 2 weeks prior to infusion. Limited data exists for vaccination while on infusion. Patients may consider getting vaccinated 4 weeks after last dose of ofatumumab. Resume 4 weeks after getting fully vaccinated |
| S1PR modulators | Receive vaccine 2 weeks prior to treatment initiation. If patient is already on the medication, vaccinate as soon as possible. |
Pharmacist Responsibilities in MS Patient Management
Pharmacists have vital responsibilities in regular clinical follow up and surveillance with the expanding arsenal of DMTs. Patients with MS need extensive education about the possible side effects and disease management (see Table 4).49 Due to the effects on the immune system, most DMTs are associated with an increased risk of infections, typically urinary tract or upper respiratory tract infections and pneumonias. In addition, pharmacists must monitor for laboratory abnormalities, opportunistic infections, autoimmune diseases, and malignancies with DMTs (see Table 5).48
Table 4. Monitoring Strategies for Adverse Effect Management49
| Drug Name | Potential adverse effect | Adverse effect management |
| Fumarates | · Gastrointestinal symptoms
· Flushing |
· Slow dose titration, from 120 mg twice daily to 240 mg twice daily over 4 weeks (instead of 7-day uptitration schedule)
· Administration with high-fat, high-protein, and low-starch food · If symptoms persist, temporary dose reduction and symptomatic treatment with antacids, promethazine may be required · Non-enteric coated aspirin up to 325 mg administered 30 minutes prior to dosing reduces the incidence or severity of flushing |
| Infusion therapies | · Infusion associated reactions (chills, flushing, fever, nausea, hives, dyspnea, and itching) and allergic reactions | · For alemtuzumab and ocrelizumab, appropriate premedication with corticosteroids, antihistamines, and antipyretics.
· Blood pressure monitoring is important, especially for alemtuzumab. |
| Interferon-beta and glatiramer acetate | · Injection site reactions | · Cold compress, premedication with NSAIDs |
| S1PR modulators (spingolimod) | · Bradycardia, atrioventricular block | · Monitor patients for six hours after initial dose, with pulse and blood pressure assessment hourly |
Table 5. Laboratory Monitoring for DMTs 27,39-47
| Medication | Required baseline | Required monitoring | Recommended additional monitoring |
| Fumarates | |||
| Dimethyl fumarate, Diroximel fumarate
Monomethyl fumarate |
CBC with differential, LFTs | CBC with differential every 6 months. | Annual LFTs |
| Monoclonal Antibodies | |||
| Natalizumab | CBC with differential, LFTs, JCV serology, brain MRI | JCV serology every 3-6 months, brain MRI every 6-12 months for JCV-seronegative patients. | CBC with differential, LFTs every 6 months. anti-natalizumab neutralizing antibodies at 3 months |
| Ocrelizumab | CBC with differential, LFTs, hepatitis panel, serum immunoglobulins | None | PPD or QFT at baseline, CBC with differential, LFTs annually, and serum immunoglobulins periodically during treatment |
| Ofatumumab | Hepatitis panel, serum immunoglobulins | None | PPD or QFT at baseline, CBC with differential, LFTs annually, and serum immunoglobulins periodically during treatment |
| Alemtuzumab | CBC with differential, creatinine, thyrotropin, ALT, AST, hepatitis panel, VZV antibodies, PPD or QFT, urinalysis, baseline skin exam, ECG | CBC with differential, creatinine, LFTs, and urinalysis every month until 48 months after last dose. Thyrotropin every 3 months until 48 months after last dose, annual skin exam. | HIV at baseline, liver function, gynecologic exam/HPV screen annually |
| Platform therapies | |||
| Interferons | CBC with differential, LFTs | CBC with differential, LFTs every 6 months | None |
| Glatiramer acetate | None | None | CBC with differential and LFTs |
| Purine analogue | |||
| Cladribine | CBC with differential, LFTs, PPD and QFT, VZV antibodies, HIV, hepatitis panel, cancer screening. Baseline MRI | CBC with differential 2 and 6 months after initiation, if lymphocyte count <200/mcL, test monthly for 6 months. Age-appropriate cancer screening. | None |
| Pyrimidine synthesis inhibitor | |||
| Teriflunomide | CBC with differential, LFTs, PPD or QFT, blood pressure | Liver function monthly for first 6 months, then every 6 months. | CBC with differential every 6 months |
| Sphingosine-1 receptor modulator | |||
| Fingolimod | CBC with differential , LFTs, ECG, VZV antibodies, fundus exam | CBC with differential, LFTs every 6 months. Fundus exam 3-4 months after initiation. | Skin exam |
| Siponimod | CYP29 genotype, CBC with differential , VZV antibodies, LFTs, ECG, fundus exam | None | CBC with differential, LFTs every 6 months |
| Ozanimod | CBC with differential , VZV antibodies, LFTs, ECG, fundus exam | None | CBC with differential and LFTs every 6 months |
ABBREVIATIONS: JCV = John Cunningham Virus (to detect PML); LFT = Liver function test; PPD = purified protein derivative (TB test); QFT = QuantiFERON-TB Gold (TB Test); VZV = varicella zoster virus
CONCLUSION
Patients with MS need lifelong therapy with DMTs. With the available medications, patients now have the convenience of oral therapies. Prevention of relapses and disability progression are the primary treatment goals. Clinicians must tailor their treatment to individual treatment needs. Women of childbearing age need extensive counseling during the prenatal, pregnancy and post-partum phase. Treatment challenges remain with a differential disease presentation in the pediatric population. Patient education and drug monitoring are profound opportunities for pharmacists.
Pharmacist Post Test (for viewing only)
This test is for viewing purposes only. If you would like to submit the test, go to the blue button at the top of the page or Test/Evaluation Site.
1. Which of the following are the three proposed immune responses that may contribute to the development of MS?
A. Antigen presentation to CD4+ lymphocytes, up-regulation of adhesion molecules, and a downstream inflammatory cascade of events.
B. Antigen presentation to CD4+ lymphocytes, up-regulation of axonal apoptosis, and an upstream inflammatory cascade of events.
C. Antigen presentation to CD4+ lymphocytes, up-regulation of adhesion molecules, and a downstream inflammatory cascade of events.
2. Researchers propose three factors influence the immune-mediated CNS injury associated with MS. What are they?
A. Environmental, genetic, and hormonal agents
B. Environmental, genetic, and infectious agents
C. Genetic, and infectious agents and inactivity
3. Among medications used for patients with MS, which of the following statements would describe alemtuzumab?
A. First line agent for high induction therapy
B. Second line after poor response to > 2 therapies
C. Only approved for inactive SPMS therapy
4. What are dimethyl fumarate’s common adverse effects?
A. Flushing and GI adverse effects
B. First-dose bradycardia and heart block
C. Progressive multifocal leukoencephalopathy
5. A patient CB calls the pharmacy and asks to speak to the pharmacist. Her pregnancy test is positive, and she has active RRMS and takes fingolimod. What should the pharmacist advise the patient?
A. Stop fingolimod for three months, then resume the fingolimod
B. Continue on fingolimod throughout the pregnancy
C. Inform her to call the provider’s office immediately.
6. The patient CB delivers a healthy baby, what would you tell her about MS in the post-partum phase?
A. In patients with active pre-pregnancy MS, relapse usually occurs within 4 months
B. In patients with active pre-pregnancy MS, relapse rates decrease in the 3 months post-partum
C. In patients with active pre-pregnancy MS, relapse rates are unpredictable and extreme
7. Which of the following is the only FDA approved agent for the treatment of pediatric MS?
A. Glatiramer acetate
B. Fingolimod
C. Interferon-beta
8. Patient AB is a newly diagnosed patient with MS. He is very anxious about his disease prognosis. His provider wants him to start ofatumumab injections. He is at the pharmacy to receive his shingles vaccine, and he tells you he plans to start the injections 7 days from vaccine administration. What should you tell him?
A. He can start ofatumumab treatment regardless of when he receives non-live vaccinations
B. He can start ofatumumab injections 7 days after vaccine administration as he planned
C. He must wait to start ofatumumab injections for 14 days from vaccine administration
9. Which of the following monoclonal antibodies has the highest risk of PML
A. Natalizumab
B. Ocrelizumab
C. Ofatumumab
10. While conducting routine follow up with a patient who takes teriflunomide, you notice she has not received her COVID booster shot. What will you tell the patient?
A. She has to stop teriflunomide for 2 weeks post vaccine administration
B. She cannot receive her booster shot while on teriflunomide
C. She can (and should) receive her booster shot while on teriflunomide
Pharmacy Technician Post Test (for viewing only)
This test is for viewing purposes only. If you would like to submit the test, go to the blue button at the top of the page or Test/Evaluation Site.
1. Which of the following accurately describes “scleroses”:?
A. Antigen presentation to CD4+ lymphocytes, up-regulation of adhesion molecules, and a downstream inflammatory cascade of events.
B. indurations or hardened areas in the brain
C. Antigen presentation to CD4+ lymphocytes, up-regulation of adhesion molecules, and a downstream inflammatory cascade of events.
2. What symptoms does Charcot’s triad include?
A. Fatigue, visual impairment, and pain
B. Dysarthria, ataxia, and tremor
C. Motor weakness, reduced mobility, and sensory loss
3. Which of the following is a problem with MS as a “silent disease”?
A. Others often notice the signs and symptoms before the affected patient does
B. The disease lays dormant for many years, and then—BOOM!—it turns deadly
C. Progression can occur with disability accrual without relapse activity
4. A patient comes in to pick up her prescription and she is visibly flushed. You ask if she rushed in or has been exercising, and she says, “No, I’ve been flushed like this since I started my new MS treatment.” Which oral drug is most likely to cause this adverse reaction?
A. dimethyl fumarate
B. glatiramer acetate
C. ocrelizumab
5. A patient CB calls the pharmacy and asks to speak to the pharmacist. Her pregnancy test is positive, and she has active RRMS and takes fingolimod. It’s very busy and the pharmacist has a backlog of prescriptions. How important is this call?
A. Not important; take a message and tell the patient to expect a call from the pharmacist within 48 hours
B. Moderately important; ask the pharmacist if you can tell the patient to keep taking the fingolimod
C. Very important; this drug is contraindicated in pregnancy, so ask the pharmacist to take the call immediately.
6. A patient has been on an injectable platform biologic for MS for several years and brings a new prescription for an oral medication. Looking forward, what are the chances that she will be nonadherent in one year?
A. 10% (1 in 10)
B. 20% (1 in 5)
C. 80% (4 in 5)
7. Which of the following is the only FDA approved agent for the treatment of pediatric MS?
A. Glatiramer acetate
B. Fingolimod
C. Interferon-beta
8. Patient AB is a newly diagnosed with MS. He is very anxious about his disease prognosis. His provider wants him to start ofatumumab injections. He is at the pharmacy to receive his shingles vaccine, and he tells you he plans to start the injections 7 days from vaccine administration. What should you tell him?
A. He can start ofatumumab treatment regardless of when he receives non-live vaccinations
B. He can start ofatumumab injections 7 days after vaccine administration as he planned
C. He must wait to start ofatumumab injections for 14 days from vaccine administration
9. Which of the following monoclonal antibodies has the highest risk of PML
A. Natalizumab
B. Ocrelizumab
C. Ofatumumab
10. While conducting routine follow up with a patient who takes teriflunomide, you notice she has not received her COVID booster shot. What will you tell the patient?
A. She has to stop teriflunomide for 2 weeks post vaccine administration
B. She cannot receiver her booster shot while on teriflunomide
C. She can (and should) receive her booster shot while on teriflunomide
References
Full List of References
References
1. Zalc B. One hundred and fifty years ago Charcot reported multiple sclerosis as a new neurological dis-ease. Brain. 2018;141(12):3482-3488.
2. Cohan SL, Hendin BA, Reder AT, et al. Interferons and Multiple Sclerosis: Lessons from 25 Years of Clinical and Real-World Experience with Intramuscular Interferon Beta-1a (Avonex). CNS Drugs. 2021;35(7):743-767
3. What is MS? National Multiple Sclerosis Society 2022. Accessed on April 16, 2022. What is MS? | National Multiple Sclerosis Society (nationalmssociety.org)
4. Villaverde-González R. Updated Perspectives on the Challenges of Managing Multiple Sclerosis During Preg-nancy. Degener Neurol Neuromuscul Dis. 2022;12:1-21. Published 2022
5. Bebo B, Cintina I, Yang W, et al. Economic burden of multiple sclerosis in 2019. Presented at ACTRIMS Forum 2022; February 24-26; West Palm Beach, FL and Virtual. Session CE3.1
6. Setayeshgar S, Kingwell E, Zhu F, et al. Persistence and adherence to the new oral disease-modifying therapies for multiple sclerosis: A population-based study. Mult Scler Relat Disord. 2019;27:364-369.
7. Loma I, Heyman R. Multiple sclerosis: pathogenesis and treatment. Curr Neuropharmacol. 2011;9(3):409-416.
8. Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implemen-tation Subcommittee of the American Academy of Neurology [published correction appears in Neurology. 2019 Jan 8;92(2):112]. Neurology. 2018;90(17):777-788
9. Stankiewicz JM, Weiner HL. An argument for broad use of high efficacy treatments in early multiple sclero-sis. Neurol Neuroimmunol Neuroinflamm. 2019;7(1):e636. Published 2019 Nov 22.
10. Olek JM, Mowry E. Initial disease-modifying therapy for relapsing-remitting multiple sclerosis in adults. In: Dashe FJ, UpToDate. UpToDate; 2020. Accessed April 12, 2022. www.uptodate.com
11. Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. European Study Group on interferon beta-1b in secondary progressive MS. Lancet. 1998;352(9139):1491-1497.
12. Milo R. Therapies for multiple sclerosis targeting B cells. Croat Med J. 2019;60(2):87-98
13. Mancinelli CR, Rossi N, Capra R. Ocrelizumab for the Treatment of Multiple Sclerosis: Safety, Efficacy, and Pharmacology. Ther Clin Risk Manag. 2021;17:765-776. Published 2021 Jul 30.
14. Bar-Or A, Grove RA, Austin DJ, et al. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study [published correction appears in Neurology. 2018 Sep 11;91(11):538]. Neurology. 2018;90(20):e1805-e1814
15. Gärtner J, Hauser SL, Bar-Or A, et al. Efficacy and safety of ofatumumab in recently diagnosed, treatment-naive patients with multiple sclerosis: Results from ASCLEPIOS I and II [published online ahead of print, 2022 Mar 10]. Mult Scler. 2022;13524585221078825
16. Rudick R, Polman C, Clifford D, et al. Natalizumab: bench to bedside and beyond. JAMA Neurol. 2013;70(2):172-182.
17. Ruck T, Bittner S, Wiendl H, Meuth SG. Alemtuzumab in Multiple Sclerosis: Mechanism of Action and Be-yond. Int J Mol Sci. 2015;16(7):16414-16439. Published 2015 Jul 20
18. Cohen JA, Coles AJ, Arnold DL, et al. CARE-MS I investigators. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 tri-al. Lancet. 2012;380(9856):1819–1828
19. Coles AJ, Twyman CL, Arnold DL, et al. CARE-MS II investigators. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 tri-al. Lancet. 2012;380(9856):1829–1839
20. Piehl F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. J In-tern Med. 2021;289(6):771-791
21. Naismith RT, Wundes A, Ziemssen T, et al. Diroximel Fumarate Demonstrates an Improved Gastrointestinal Tolerability Profile Compared with Dimethyl Fumarate in Patients with Relapsing-Remitting Multiple Sclerosis: Results from the Randomized, Double-Blind, Phase III EVOLVE-MS-2 Study. CNS Drugs. 2020;34(2):185-196
22. Wynn D, Lategan TW, Sprague TN, Rousseau FS, Fox EJ. Monomethyl fumarate has better gastrointestinal tolerability profile compared with dimethyl fumarate. Mult Scler Relat Disord. 2020;45:102335
23. Miller AE. An updated review of teriflunomide's use in multiple sclerosis. Neurodegener Dis Manag. 2021;11(5):387-409
24. McGinley MP, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis and other condi-tions [published correction appears in Lancet. 2021 Sep 25;398(10306):1132]. Lancet. 2021;398(10306):1184-1194
25. Roy R, Alotaibi AA, Freedman MS. Sphingosine 1-Phosphate Receptor Modulators for Multiple Sclero-sis. CNS Drugs. 2021;35(4):385-402
26. Chun J, Hartung HP. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin Neuro-pharmacol. 2010;33(2):91-101
27. Mavenclad[package insert] Merck KGaA; 2012
28. Dobson R, Hellwig K. Use of disease-modifying drugs during pregnancy and breastfeeding. Curr Opin Neurol. 2021;34(3):303-311
29. Steingo B, Al Malik Y, Bass AD, et al. Long-term efficacy and safety of alemtuzumab in patients with RRMS: 12-year follow-up of CAMMS223. J Neurol 2020; 267:3343–3353.
30. Brola W, Steinborn B. Pediatric multiple sclerosis - current status of epidemiology, diagnosis and treat-ment. Neurol Neurochir Pol. 2020;54(6):508-517.
31.Banwell B, Reder AT, Krupp L, et al. Safety and tolerability of interferon beta-1b in pediatric multiple sclero-sis. Neurology. 2006;66(4):472-476.
32. Tenembaum SN, Banwell B, Pohl D, et al. Subcutaneous interferon Beta-1a in pediatric multiple sclerosis: a retrospective study. J Child Neurol. 2013;28(7):849-856.
33. Chitnis T, Arnold DL, Banwell B, et al. Trial of Fingolimod versus Interferon Beta-1a in Pediatric Multiple Sclerosis. N Engl J Med. 2018;379(11):1017-1027
34. Waubant E, Banwell B, Wassmer E, et al. Clinical trials of disease-modifying agents in pediatric MS: Oppor-tunities, challenges, and recommendations from the IPMSSG [published correction appears in Neurology. 2019 Oct 1;93(14):647]. Neurology. 2019;92(22):e2538-e2549.
35.Otero-Romero S, Ascherio A, Lebrun-Frénay C. Vaccinations in multiple sclerosis patients receiving disease-modifying drugs. Curr Opin Neurol. 2021;34(3):322-328.
36. Bar-Or A, Calkwood JC, Chognot C, et al. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: The VELOCE study. Neurology. 2020;95(14):e1999-e2008
37. Achiron A, Mandel M, Dreyer-Alster S, et al. Humoral immune response in multiple sclerosis patients follow-ing PfizerBNT162b2 COVID19 vaccination: Up to 6 months cross-sectional study. J Neuroimmunol. 2021;361:577746
38. Dreyer-Alster S, Menascu S, Mandel M, et al. COVID-19 vaccination in patients with multiple sclerosis: Safe-ty and humoral efficacy of the third booster dose. J Neurol Sci. 2022;434:120155.
39.Adakveo [package insert]. Novartis Pharmaceuticals Corporation;2019
40. Ocrevus[package insert]. Genentech Inc;2017
41. Kesimpta[package insert]. Novartis Pharmaceuticals Corporation; 2020
42. Lemtrada[package insert]. Genzyme Corporation; 2017
43. Gilenya[package insert]. Novartis Pharmaceuticals Corporation; 2012
44. Zeposia[package insert]. Celgene Corporation; 2020
45. Ponvory[package insert] Janssen Pharmaceutical Companies; 2021
46. Mayzent[package insert] Novartis Pharmaceuticals Corporation;2019
47. Ponvory [package insert] Janssen Pharmaceuticals Inc; 2021
47. COVID-19 VACCINE GUIDANCE FOR PEOPLE LIVING WITH MS. National Multiple Sclerosis Society. 2022. Accessed on April 16, 2022. COVID-19 Vaccine Guidance for People Living with MS | National MS Society | National Multiple Sclerosis Society.
48. Moiola L, Rommer PS, Zettl UK. Prevention and management of adverse effects of disease modifying treat-ments in multiple sclerosis. Curr Opin Neurol. 2020;33(3):286-294.
49. Fyfe I. More CLARITY on long-term benefits of cladribine. Nat Rev Neurol. 2021;17(12):726.
50. Sriwastava S, Kataria S, Srivastava S, et al. Disease-modifying therapies and progressive multifocal leu-koencephalopathy in multiple sclerosis: A systematic review and meta-analysis. J Neuroimmunol. 2021;360:577721.
51. Hartung DM. Economics of Multiple Sclerosis Disease-Modifying Therapies in the USA. Curr Neurol Neuro-sci Rep. 2021;21(7):28