Learning Objectives
| Explain the benefits to women, children, and society when contraceptives are easily accessible |
|
|
|
|
|
|

Release Date:
Release Date: November 27, 2024
Expiration Date: November 27, 2027
Course Fee
Pharmacist $40
There is no funding for this CPE activity.
ACPE UANs
Pharmacist: 0009-0000-24-054-H01-P
Session Code
Pharmacist: 24BC54-CBA36
Accreditation Hours
4.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-24-054-H01-P will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Kelsey Giara, Pharm.D.
UConn Adjunct Faculty
University of Connecticut
Storrs, CT
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Dr. Giara has no financial relationships with ineligible companies.
ABSTRACT
This continuing education module was developed to meet the State of Connecticut pharmacist contraceptive prescribing requirements. Pharmacists who wish to prescribe contraceptives in this state can complete this activity. They should note that after reading the content, they must take an 80-question post-test and pass with a score of at least 80%. They should be prepared to use the materials included as Appendices. They should also visit the state’s web page on this topic (https://portal.ct.gov/dcp/drug-control-division/drug-control/drug-control---pharmacist-contraceptive-prescribing?language=en_US) where they will find additional documents, including the link to the questionnaires for prescribing hormonal contraceptives and emergency contraceptives.
CONTENT
Content
INTRODUCTION
More than 72 million individuals of reproductive age (15 to 49 years old) live in the United States (U.S.), and about 43 million of them are at risk of unintended pregnancy.1 This means they are sexually active and could experience unwanted pregnancy if they and their partners fail to use contraceptives consistently and correctly.
Here is a striking but under-reported fact: about one in two pregnancies in the U.S. is unintended (i.e., mistimed or unwanted at the time of conception).2 Compared to intentional pregnancies, people experiencing unintended pregnancy experience more mental health problems, have less stable romantic relationships, and sometimes delay initiation of prenatal care.3 Ideally, those who unintentionally conceive should ideally be in good health and ready to care for a new child, but sometimes that is not the case.
Children born as a result of unintended pregnancies are at an elevated risk of experiencing both mental and physical health challenges and are more likely to struggle in school.3 While the overall rate of unintended pregnancies is on the decline, disparities based on factors such as race/ethnicity, age, income, and education level persist.3
It is crucial to implement interventions that promote the use of contraception methods to prevent unintended pregnancies. While over the counter (OTC) options for pregnancy prevention exist, hormonal methods requiring a prescription (pill, patch, ring, and injection) are more effective than OTC products, withdrawal, or fertility-awareness methods for pregnancy prevention.4 Recent laws make it possible for pharmacists to prescribe hormonal contraceptives in some states, increasing access to these more effective therapies.
Note that this activity will employ the terms "woman/women" to align with the biological expectations of ovulation.
Understanding the Menstrual Cycle
The length of a woman’s menstrual cycle is a commonly misunderstood concept. While most people consider a natural 28-day cycle “normal,” this is only true for about 13% of women.5 The first day of menstrual bleeding is considered cycle day 1 and cycles range from about 21 to 40 days in length.
Hormone levels regulate the menstrual cycle. The pituitary gland produces luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which promote ovulation and release of estrogen and progesterone from the ovaries.6 This hormone fluctuation splits the menstrual cycle into three phases; note that the timeframes described below are approximate length based on a 28-day cycle6:
- Follicular phase (before egg release)
- Lasts from cycle day 1 to ovulation
- FSH levels rise to recruit a small group of follicles for growth and development
- Between days 5 to 7, one follicle dominates and secretes estradiol to stop menstrual flow
- Ovulatory phase (egg release)
- Occurs on or around day 14
- Sustained FSH levels cause an LH surge about 28 to 32 hours before the dominant follicle ruptures, also known as ovulation, releasing the mature oocyte (egg)
- Luteal phase (after egg release)
- Lasts from ovulation to the last day of the cycle
- Remaining luteinized (matured) follicles become the corpus luteum, which produces progesterone to prepare the uterus for embryo (fertilized egg) implantation
Following the luteal phase, if no egg is fertilized or the egg does not implant, the corpus luteum degenerates after 14 days, estrogen and progesterone levels drop, and a new menstrual cycle begins.6 However, if a fertilized embryo is implanted, the cells around the embryo produce human chorionic gonadotropin (HCG). HCG maintains the corpus luteum, which continues to produce progesterone until the fetus can produce its own hormones. Pregnancy tests detect an increase in HCG in the blood or urine, indicating a fertilized embryo is present.6
PHARMACY-BASED HORMONAL AND EMERGENCY CONTRACEPTIVES
Contraception refers to the strategies employed to prevent pregnancy after sexual intercourse, which can be categorized into two main approaches7:
- Inhibiting the encounter of viable sperm with a mature ovum, achieved through methods such as barriers or ovulation prevention
- Preventing the implantation of a fertilized ovum in the endometrium, accomplished through methods that create an unfavorable uterine environment
It is crucial for sexually active individuals to be well-informed about the various contraceptive options available. This knowledge is essential in assisting patients in effectively preventing unintended pregnancies.
This activity will only discuss the birth control methods that pharmacists are directly involved in prescribing.
Hormonal Contraceptive Basics
As the name implies, hormonal contraceptives employ hormones—specifically, progestins and estrogens—to prevent pregnancy.7 Hormonal contraceptives do not protect patients from sexually transmitted infections, including human immunodeficiency virus, and this is a point that pharmacists need to stress at every visit. Estrogens’ role in birth control is to stabilize the endometrial lining and provide cycle control. However, estrogens also suppress FSH release from the pituitary gland to help block the LH surge and prevent ovulation. Progestins provide most contraceptive effect. They block the LH surge, which inhibits ovulation. Progestins also thicken cervical mucus to7
- prevent sperm penetration
- slow tubal motility
- delay sperm transport
- induce endometrial atrophy (thinning), reducing its receptivity to embryo implantation
Achieving the right balance between progestogens and estrogens is vital in hormonal contraceptives. Some hormonal contraceptives contain only a progestin, while others combine an estrogen and a progestin.7 Importantly, estrogen alone—or “unopposed estrogen”—does not protect against pregnancy and pose significant safety concerns. Patients with an intact uterus who take unopposed estrogen are at risk of cancer, endometrial hyperplasia, polyps, endometriosis, and adenomyosis.8
PAUSE AND PONDER: What is the difference between a CHC, a COC, and a POP?
Combined hormonal contraceptives (CHCs)—any contraceptive containing both an estrogen and progestin—are not appropriate for women7
- older than 35 years who smoke
- with obesity (body mass index 30 or greater)
- with untreated hypertension (greater than 160/100 mm Hg)
- with migraines (especially with aura)
- at risk for deep vein thrombosis
The estrogen component of most CHCs is ethinyl estradiol.7 Many different progestins of differing androgenicity and similarity to testosterone exist, but no evidence suggests that a particular progestin is superior to others. Traditionally, experts classified progestins into “generations” based on parent compound and decade of development, but data shows this is not clinically useful.7
A woman can start CHCs at any time during her cycle if it is reasonably certain that she is not pregnant.9 If a patient starts CHCs within the first five days of menstrual bleeding, no additional protection is needed, but if it has been longer than five days from the start of menses, she should abstain from intercourse or use backup contraception for the next seven days.9 Table 1 outlines missed dose guidance for all contraceptive types.
Table 1. Missed Dose Guidance for Contraceptives7,10,11
| Missed Dose/Failure | Guidance |
| COCs: 1 pill late (< 24 hours overdue) or missed (24 to < 48 hours overdue) | · Take the late/missed pill ASAP
· Continue taking remaining pills at the same time (even if 2 doses in 1 day) · No back-up contraception needed · Consider emergency contraception if previous late/missed dose in same cycle or in the last week of the previous cycle |
| COCs: ≥ 2 consecutive pills missed (≥ 48 hours have passed since last pill) | · Take the most recently missed pill ASAP
· Discard any other missed pills · Continue therapy as usual · Use back-up contraception (e.g., condoms) or remain abstinent until they’ve taken hormonal pills for 7 consecutive days · If in last week of hormonal pills, skip placebo interval and start new pack immediately · Consider emergency contraception if pills missed in 1st week and unprotected intercourse occurred |
| POP: more than 3 hours late | · Take missed pill ASAP, then go back to regularly scheduled time
· Use back-up contraception until POP taken consistently for at least 48 hours · If vomiting occurs soon after taking, use back-up contraceptive for at least 48 hours |
| Transdermal patch: partially or completely detached | · Reapply ASAP
· If no longer sticky or becomes dirty, use a new patch (do not use supplemental wraps or adhesives) · If detached ≥ 24 hours, may no longer be protected from pregnancy; stop the current contraceptive cycle and start a new one (use back-up contraception for at least 1 week) · If unsure how long it was detached, treat it as if it was ≥ 24 hours |
| Transdermal patch: forget to change patch | · At start of a patch cycle (week 1/day 1), apply a patch as soon as possible; this becomes the new “patch change day” and back-up contraception is needed for 1st week
· In middle of a patch cycle (week 2/day 8 or week 3/day 15) < 48 hours late, apply new patch immediately; no change in “patch change day” and no back-up needed · In middle of a patch cycle (week 2/day 8 or week 3/day 15) ≥ 48 hours late, stop current cycle and start a new one with a new patch; this is new “patch change day” and should use back-up for 1 week |
| EE/E vaginal ring: falls out or removed ≥ 3 hours | · Weeks 1 or 2: use back-up contraception until ring is in place for 7 consecutive days
· Week 3: discard ring and either (1) insert new ring immediately to start next 3-week use or (2) insert a new ring ≤ 7 days from removal (only if ring was in for 7 consecutive days before removal) · Always use back-up contraception until the ring has been placed for 7 consecutive days · If removed < 3 hours, efficacy is not affected; rinse ring with cool/lukewarm water and reinsert ASAP |
| SAEE vaginal ring: falls out or removed ≥ 2 hours | · Rinse ring with cool/lukewarm water and reinsert ASAP
· Use back-up contraception until the ring has been placed for 7 consecutive days · If removed < 2 hours, efficacy is not affected |
ASAP, as soon as possible; COC, combined oral contraceptive; EE/E, ethinyl estradiol/etonogestrel; POP, progestin-only pill; SAEE, segesterone acetate/ethinyl estradiol.
Combined Oral Contraceptives
Combined oral contraceptives (COCs)—meaning oral products containing both an estrogen (e.g., ethinyl estradiol) and a progestin (e.g., norethindrone, levonorgestrel, norgestimate)—come in many forms. COCs are about 91% effective, meaning that 9 of 100 women will become pregnant in a year with typical use.12 A major distinction in product selection is monophasic versus multiphasic7:
- Monophasic COCs contain the same amounts of estrogen and progestin for 21 days, followed by seven days of placebo
- Multiphasic COCs—including bi- and triphasic regimens—contain variable amounts of estrogen and progestin for 21 days, also followed by a 7-day placebo phase
Monophasic and multiphasic COCs boast similar safety and efficacy profiles, so product selection relies on hormonal content, patient-preference, and coexisting conditions.7 Women should typically initiate a COC containing 35 mcg or less of ethinyl estradiol and less than 0.5 mg of norethindrone (or an equivalent).7 Estradiol levels control the incidence of breakthrough bleeding. Few patients require doses of ethinyl estradiol greater than 35 mcg daily to prevent breakthrough bleeding. While some clinicians advocate for starting patients at the lowest possible estradiol dose to minimize risks, data suggests that 10 to 20 mcg of ethinyl estradiol daily is no safer than the 35-mcg dose and lower doses are associated with more breakthrough bleeding.7
Monophasic COCs are preferred over multiphasic upon initiation, as adverse effects (AEs) are easier to identify and manage. Monophasic COCs also allow for easy cycle extension (continuing the active moiety to bypass a period, an indication for which pharmacists are not authorized to prescribe) by simply skipping the placebo week and starting the next pack of active pills. When attempting this with multiphasic regimens, the variation in drug levels between phases often leads to breakthrough bleeding.7
Extended- and continuous-cycle COCs contain 84 days of active hormone tablets followed by seven days of inactive tablets, which may be more convenient with fewer AEs.7 Extended-cycle COCs are commercially available. Of note, some patients skip the 7-day placebo week of monophasic 28-day COCs to mimic extended-cycle products, but pharmacists are only legally authorized to prescribe hormonal contraceptives as indicated. Continuous-cycle COCs shorten the pill-free interval (e.g., two to four days versus seven days), thus reducing period-related symptoms. Patients using extended- and continuous-cycle regimens have fewer menstrual cycles annually, which is helpful for women with severe premenstrual syndrome (PMS), dysmenorrhea (menstrual cramps), and menstrual migraines.
Progestin-Only Pills
Progestin-only pills (POPs)—“minipills”—contain 28 days of active hormone (norethindrone or drospirenone) per cycle.7 They are generally less effective than COCs and associated with irregular, unpredictable menstrual bleeding. Patients must take POPs at approximately the same time (within three hours) every day for effective pregnancy prevention. The progestin dose in POPs is about one-third of that in COCs, resulting is less consistent suppression of ovulation. This leaves women at greater risk of breakthrough bleeding and ectopic pregnancy—pregnancy outside the uterus—because women on POPs often continue to ovulate regularly.7
Despite being less effective, POPs are appropriate for certain women. Postpartum women, for example, can experience hypercoagulability, and should avoid CHCs for at least 30 to 42 days postpartum due to risk of venous thromboembolism. Therefore, those who take contraceptives commonly take POPs.7 Women who breastfeed should also avoid CHCs, as the estrogen component can affect lactation, making POPs a better option.
POPs can be started at any time during a woman’s cycle if it is reasonably certain she is not pregnant.9 If a patient starts POPs within the first five days of menstrual bleeding, she need not use additional protection , but if it has been longer than five days from the start of menses, she should abstain from intercourse or use backup contraception for the next two days.9
ORAL CONTRACEPTIVE TAKEAWAYS:
- COCs: Initiating a monophasic formulation containing 30 to 35 mcg of ethinyl estradiol and less than 0.5 mg of norethindrone (or an equivalent) offers the best chance of establishing a consistent menstrual pattern without raising AE risk. If patients experience estrogen- or progesterone-related AEs (listed in Table 2), dose adjustment is warranted.
- Extended- or continuous cycle: Patients have fewer menstrual cycles each year, making them ideal for patients with severe PMS, dysmenorrhea, or menstrual migraines.
- POPs: Less effective than COCs, but appropriate for patients within 42 days postpartum and women who breastfeed, as estrogen can affect lactation.
Table 2. Hormone-Related Adverse Effects13
| Too Much | Not Enough | |
| Estrogen | · Nausea
· Breast tenderness · Weight gain · Headaches · Menstruation changes |
· Vasomotor symptoms (night sweats, hot flashes)
· Early cycle (days 1-9) breakthrough bleeding or spotting · Amenorrhea |
| Progestins | · Breast tenderness
· Headache · Fatigue · Mood changes (depression, irritability) · Weight gain · Acne/oily skin · Hirsutism |
· Dysmenorrhea (painful periods)
· Menorrhagia (heavy menstrual bleeding) · Late cycle (days 10-21) breakthrough bleeding or spotting |
Non-Oral Hormonal Contraceptives
Some patients—particularly those who struggle with daily adherence to oral therapies—may benefit from alternative delivery mechanisms administered less frequently, including transdermal patch, vaginal ring, and injectable contraceptives.
The only transdermal CHC patch available in the U.S. contains ethinyl estradiol and norelgestromin (norgestimate’s active metabolite). The transdermal patch has comparable efficacy to COCs.12 It may be less effective, however, for patients weighing more than 198 lbs (90 kg).14 Patients apply the patch to the abdomen, buttocks, upper torso, or upper arm at the beginning of the menstrual cycle, avoiding areas where the patch could be rubbed by tight clothing.14 Patients replace the patch once weekly for 3 weeks, followed by a patch-free week. The patch is formulated to release hormones for nine days, allowing a 48-hour grace period for adherence.14
The CHC patch’s adverse effects are similar to those of COCs, but some patients experience application-site reactions. Patients can prevent these reactions by rotating application sites. Dysmenorrhea and breast discomfort are also possible, as the patch causes higher estrogen exposure compared to COCs.14
Vaginal ring contraceptives offer excellent cycle control, as patients can insert and remove them, and fertility returns rapidly after removal.15 Two vaginal ring contraceptives are available:
- an ethinyl estradiol/etonogestrel (EE/E) ring that patients replace monthly
- a segesterone acetate/ethinyl estradiol (SAEE) ring that patients replace yearly
The EE/E vaginal ring delivers 0.015 mg of ethinyl estradiol and 12 mcg of etonogestrel (desogestrel’s active metabolite) every 24 hours.16 Estrogen exposure with the EE/E ring is lower than that associated with COCs, so incidence of estrogen-related adverse effects is also decreased. Local reactions, like vaginal irritation and discharge, are more common.16 The SAEE vaginal system is slightly larger in diameter than the EE/E ring and contains two drug reservoirs delivering 0.15 mg and 13 mcg of segesterone and ethinyl estradiol, respectively, every 24 hours.15,10 The SAEE ring’s most common adverse effects are headache/migraine, nausea, vomiting, vaginal infections, abdominal pain, dysmenorrhea, vaginal discharge, urinary tract infection, breast discomfort, bleeding irregularities, diarrhea, and genital itching.10
To insert a vaginal contraceptive ring, patients should compress the ring between the thumb and index finger, then push the ring into the vagina.16,10 There is no danger of inserting too far; the cervix will prevent the ring from traveling up the genital tract. Additionally, precise ring placement is not an issue, as the hormones are absorbed anywhere in the vagina. Patients should leave the ring in place for three weeks, then remove it for one week.
If using the EE/E monthly ring, patients should discard the ring (but not flush it down the toilet) after removal and insert a new one on the same day of the week as the previous cycle.16 Alternatively, the same SAEE ring can be used for up to one year. During the ring-free week, patients should store the SAEE ring only in the provided case away from children, pets, and extreme temperatures.15,10 They should also wash the ring after removal and again before reinsertion using mild soap and water and pat dry with a clean cloth or paper towel. Patients should never use the same SAEE ring for more than 13 menstrual cycles.15,10
If a vaginal contraceptive ring is removed from the vagina, intentionally or otherwise, no backup contraception is needed if the patient reinserts the ring within three hours for the EE/E ring and two hours for the SAEE ring.16,10 If the ring remains out of the vagina for longer than these recommended time periods, backup contraception (e.g., male condoms, spermicide) is recommended for seven days after ring reinsertion. Patients should also avoid oil-based lubricants, as these can decrease the effectiveness of vaginal contraceptive rings.
Both vaginal ring systems carry risks for toxic shock syndrome (TSS), a rare, potentially life-threatening vital organ failure caused by bacterial infection.16,10 Items that remain in the vagina for an extended period of time are implicated in TSS because bacteria can be trapped in the vagina and enter the uterus via the cervix or objects in the vagina can also cause tiny cuts through which bacteria can enter the bloodstream. Advise patients to seek medical attention if they experience signs/symptoms of TSS17:
- nausea or vomiting
- sudden high fever and chills
- watery diarrhea
- rash resembling a bad sunburn or red dots
- dizziness, light-headedness, or fainting
- hypotension
- red eyes (conjunctivitis)
- peeling on the soles of feet or palms of hands
Depo-medroxyprogesterone acetate (DMPA) is a longer-lasting injectable contraceptive injected every three months either intramuscularly into the gluteal or deltoid muscle or subcutaneously into the abdomen or thigh.18 This eliminates daily adherence concerns. DMPA is about 94% effective with typical use.9 Note that Connecticut law prohibits pharmacists from administering DMPA injections without a collaborative practice agreement, but patients may self-administer DMPA subcutaneously if desired and indicated.
Injection timing is somewhat flexible. Early DMPA injection is safe if women cannot follow routine intervals, and patients can inject up to two weeks late without requiring back-up contraception.18 Women who are more than two weeks late, however, should use back-up contraception for seven days after receiving the injection. Return to fertility may be delayed six to 12 months after discontinuation, so DMPA is not recommended for women desiring pregnancy in the near future.18
DMPA’s most common adverse effects are weight gain, decreased bone mineral density, and bleeding irregularities (e.g., spotting, prolonged bleeding, amenorrhea).18 DMPA carries a Boxed Warning indicating patients should not use the drug for more than two years due to bone mineral density loss, which may be irreversible.18 Patients should only use DMPA for more than two years if all other contraceptive methods are inadequate. Ensure patients are adequately trained to self-inject DMPA before leaving the pharmacy.
Emergency Contraceptives
Two oral emergency contraceptives (ECs) are currently available: a single dose of progestin (levonorgestrel 1.5 mg) or an anti-progestin (ulipristal acetate 30 mg).7 Levonorgestrel is available over the counter, while ulipristal requires a prescription.19,20 Neither of these is abortifacient (i.e., they do not end an existing pregnancy), rather they work by blocking or delaying ovulation.
Women should take oral EC as soon as possible following unprotected intercourse, levonorgestrel within 72 hours and ulipristal acetate within five days.19,20 Repeat levonorgestrel use shows no serious adverse effects, but studies have not examined repeat use of ulipristal acetate.7 Upon prescribing emergency contraception, pharmacists should evaluate women for long-term contraceptive eligibility to prevent repeat use of EC.
EC may alter the next expected menses.19,20 Patients whose cycles are delayed more than one week should test for pregnancy. Additionally, women who become pregnant after using EC who experience lower abdominal pain should be evaluated for ectopic pregnancy (pregnancy occurring outside the uterus, most often in the fallopian tube).19,20
PAUSE AND PONDER: Where can you find the living document called the United States Medical Eligibility Criteria (U.S. MEC) for Contraceptive Use and how often should you review its contents? (Note that some questions in the post-test will require you to access this document, so you must review it thoroughly.)
ELIGIBILITY AND PRESCRIBING
CDC Eligibility Criteria
The CDC publishes the United States Medical Eligibility Criteria (U.S. MEC) for Contraceptive Use to guide safe use of contraceptive methods for women with various medical conditions and other characteristics.21 The most current version of these guidelines (as of February 2024) can be found at https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html. Appendix A of this activity includes the entire U.S. MEC and the 2020 update. The CDC has also created a summary chart, included in Appendix B, and pharmacists should note that the summary chart is a convenience that does not replace a responsibility to access the entire document when necessary. Pharmacists actively prescribing hormonal contraceptives should regularly monitor for updates to this living document.
The U.S. MEC includes recommendations for contraceptive use based on patient characteristics or medical conditions.21 While many off label uses exist for contraceptives, these CDC recommendations apply only to these products’ indicated use to prevent pregnancy.
Four categories of medical eligibility criteria for contraceptive use exist within the U.S. MEC:
- Category 1: conditions for which no restrictions exist for use of the contraceptive method
- Category 2: conditions for which the advantages of using the method generally outweigh the theoretical or proven risks; the method can generally be used, but careful follow-up might be required
- Category 3: conditions for which the theoretical or proven risks usually outweigh the advantages of using the method; use is not recommended unless other more appropriate methods are not available or acceptable, so condition severity and the availability, practicality, and acceptability of alternative methods should be considered, and careful follow-up is required
- Category 4: conditions that represent an unacceptable health risk if the contraceptive method is used
Be mindful that provision of a contraceptive method to a woman with a condition classified as category 3 requires careful clinical judgement and access to clinical services that may be unavailable to pharmacists. Referral may be needed. Pharmacists should never prescribe a hormonal contraceptive method to a patient with a category 4 health condition related to its use.
Pharmacists should also take note of whether continuation criteria exist for the product prescribed.21 Continuation criteria is clinically relevant when a medical condition develops or worsens during use of a contraceptive method. When risk categories differ for initiation and continuation, the differences are noted in the Initiation and Continuation columns. When these distinctions are not indicated, the category is the same for initiation and continuation of use.21
Additionally, these categories only concern safety, but many other factors must be considered when choosing a contraceptive method. Classification as a category 1 means that the method can be used with no regard to safety but does not necessarily mean that method is the best choice for that patient. Consider other factors, including effectiveness, availability, and acceptability.
Determining Pregnancy Status
The Centers for Disease Control and Prevention (CDC) recognizes that routine pregnancy testing for women is not necessary before the initiation of contraception in all cases. Based on clinical judgment, healthcare providers can omit a pregnancy test if a woman has no signs or symptoms of pregnancy and meets any of the following criteria21:
- Is fewer than seven days after the start of normal menses
- Has not had sexual intercourse since the start of last normal menses
- Has correctly and consistently used a reliable contraception method
- Is fewer than seven days after spontaneous or induced abortion
- Is within four weeks postpartum
- Is fully or nearly-fully breastfeeding (exclusively or at least 85% of feeds), amenorrhoeic, and less than six months postpartum
Screening Documents Simplify the Process
A prescribing pharmacist must assist the patient in completing a screening document for hormonal contraceptives or emergency contraceptives, as applicable. These and all documents related to prescribing of hormonal contraceptives by pharmacists in the state of Connecticut are available at the Department of Consumer Protection’s (DCP) website: https://portal.ct.gov/DCP/Drug-Control-Division/Drug-Control/Drug-Control---Pharmacist-Contraceptive-Prescribing. Prescribing pharmacists are responsible for ensuring their pharmacies use the most current version of all screening documents at all times.
When an individual requests a hormonal contraceptive, pharmacists must first determine the patient’s age. If the patient is 18 years or older, the pharmacist may continue to prescribe with this guidance, but if the patient is younger than 18 years, the pharmacist may only issue a prescription upon confirming the patient has previously been prescribed a contraceptive by another provider through one of the following means:
- With the patient’s permission, contact the office or clinic where the patient visited a healthcare provider via telephone, facsimile, or shared health record system
- With the patient’s permission, contact the pharmacy that previously dispensed a contraceptive prescription to the patient via telephone, facsimile, or shared health record system
- Other acceptable documentation or evidence that demonstrates the patient has received prescription contraceptives (e.g., visit summary from the clinic that prescribed it, old prescription package/label)
The pharmacist must keep an electronic or written record of the action taken to confirm prior prescription for a minimum of 3 years. Whether prescribing a continuation of hormonal contraceptive therapy or initiating a new one, confirm the patient has been seen by a provider within the last 3 years either through written documentation (e.g., a visit summary) or contacting the office or clinic with the patient’s permission. Without this confirmation, pharmacists may not prescribe hormonal contraception.
Individuals with obesity (body mass index 30 kg/m2 or greater) and patients using drugs that inhibit CYP3A4 (e.g., bosentan, carbamazepine, felbamate, griseofulvin, oxcarbazepine, phenytoin, rifampin, St. John’s wort, topiramate, efavirenz, lumacaftor) may experience decreased efficacy with EC.21 Patients ineligible for EC prescribing include those who
- are confirmed pregnant (though no harm to the woman, the course of her pregnancy, or the fetus if EC is inadvertently used is known to exist)
- have undergone bariatric surgery that may affect EC absorption (e.g., Roux-en-Y gastric bypass, biliopancreatic diversion); an emergency intrauterine device may be more appropriate
If a patient seeking EC is deemed ineligible for prescribing, the pharmacist should not prescribe the EC, refer the individual to a primary care provider (PCP), and document the reason(s) for refusal on the screening documents. If the patient has no PCP, the pharmacist should provide information regarding local providers.
Hormonal Contraceptive Screening and Prescribing
Upon determining patient eligibility, have the patient complete the Connecticut Hormonal Contraceptive Self- Screening Questionnaire found on the DCP website. The questionnaire addresses
- patient demographics (e.g., insurance status, allergies, preferences)
- background information (e.g., last menstrual period, history of contraceptive use)
- medical history (e.g., smoking status, preexisting conditions)
- pregnancy status
Review this screening tool with the patient and clarify responses if needed. If a patient is requesting CHCs or they are recommended, measure and record the patient’s seated blood pressure. If the patient does not complete the questionnaire, a pharmacist cannot issue the prescription. Pharmacists should keep the completed questionnaire on file for at least three years. Patients must fill out a new questionnaire at least once every 12 months, but pharmacists can request this more frequently if desired.
Pharmacists should then use patient questionnaire responses to follow the Standard Procedures Algorithm for Connecticut Pharmacist Prescribing of Contraceptives found on the DCP website. This algorithm, with its clinical assessment sections summarized in Figure 1, assists in screening for red flags requiring provider referral. Pharmacists should access the complete document on the DCP website before attempting to take the post-test.
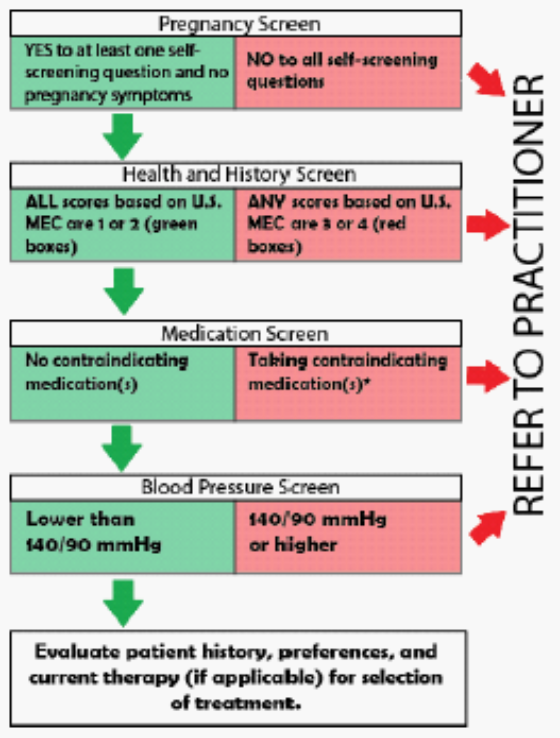
Figure 1. Simplified Assessment Sections of the Algorithm for Contraceptive Prescribing
*Anticonvulsants, antiretrovirals, antimicrobials, barbiturates, herbs and supplements, including but not limited to: carbamazepine, felbamate, phenobarbital, lamotrigine, oxcarbazepine, ritonavir, primidone, griseofulvin, St. Jonh’s wort, topiramate, phenytoin, lumacaftor/ivacaftor, and rifampin/rifabutin.
Pharmacists should pay special attention to steps 6 and 7 of the Standard Procedures Algorithm (and take a moment to access it now). It describes counseling points on starting hormonal contraception, managing expected and unexpected side effects, and appropriate adherence. Not that Step 7 is critical! Women need to be reminded about routine healthcare and sexually transmitted infection prevention. And the pharmacist’s job isn’t done until the paperwork is filed.
If hormonal contraceptives are not clinically appropriate based on the treatment algorithm, the pharmacist should refer the patient to a practitioner, not prescribe the hormonal contraceptive, and document the reason(s) for refusal on the screening documents. The State of Connecticut provides a Pharmacist Referral and Visit Summary template that may be used for this purpose. If hormonal contraceptives are clinically appropriate, pharmacists may prescribe a total of no more than 12 months including initial filling of the prescription along with refills. Refills may be transferred to another pharmacy if desired, as a pharmacy that does not have a prescribing pharmacist may dispense a prescription written by a prescribing pharmacist. Pharmacies may not, however, fill prescriptions written by pharmacists authorized to prescribe in other states but not in Connecticut.
Emergency Contraceptive Screening and Prescribing
To be eligible for a self-administered EC prescription, an individual must complete the Connecticut Emergency Contraception Self-Screening Questionnaire found on the DCP website indicating that the last day of unprotected intercourse was within the previous five days (120 hours). Pharmacies may create and use an electronic version of this self-screening tool if the collection of patient information and assessment process is at minimum identical to the state-provided questionnaire. The pharmacist must review the screening tool with the patient, clarify responses if needed, and measure and record the patient’s seated blood pressure.
Pharmacists should consider the following when choosing between levonorgestrel and ulipristal acetate for EC:
- Levonorgestrel may be less effective than ulipristal acetate for women who weigh more than 165 lbs
- Levonorgestrel may be preferable for patients who need EC due to missed or late administration of existing hormonal contraception
- Starting hormonal contraceptives immediately after taking ulipristal acetate (within 5 days) may make it ineffective
- Insurance may still cover OTC levonorgestrel if a pharmacist prescribes the product
- Ulipristal acetate is more effective than levonorgestrel if more than 72 hours have passed since the last day of unprotected intercourse
Prescriptions for EC may not have any refills. Upon prescribing EC, counsel patients on the product’s proper use and potential adverse effects and provide written educational materials. The pharmacist must also notify the patient’s PCP and obstetrician/gynecologist (OB/GYN) with the patient's consent. If the patient does not have a PCP, the pharmacist should counsel the patient regarding the benefits of establishing such a relationship and, upon request, provide information regarding local providers. The pharmacist should also counsel the patient regarding the importance of preventive care, including routine well-woman visits, testing for sexually transmitted infections, and pap smears. Additionally, consider whether patients should also be evaluated for ongoing hormonal contraception, especially if they visit the pharmacy repeatedly for EC.
STICKING TO THE LAW
PAUSE AND PONDER: Are you ready for pharmacy-based contraceptive prescribing? Where will you maintain your documentation?
Documentation and Recordkeeping
Pharmacies must maintain appropriate records of hormonal contraceptive and EC prescribing:
- All completed screening documents must be maintained at the prescribing pharmacy in the same manner as the prescription itself for at least three years.
- All records created as part of the prescribing process must be maintained for at least three years and be readable retrievable and provided to DCP within 48 hours
Prohibited Acts
A prescribing pharmacist shall not
- prescribe any hormonal contraceptive or EC in an instance where the screening document for hormonal contraceptive or screening document emergency contraceptives indicates that referral to a practitioner is clinically appropriate
- prescribe any hormonal contraceptive or EC without a completed screening document for hormonal contraceptive or completed screening document for emergency contraceptive, as applicable
- issue a prescription for a total supply period exceeding 12 months based on the directions of use provided on the prescription
- prescribe any hormonal contraceptive or EC outside of the approved use stated in the product’s FDA-approved package insert
- prescribe a medical device, with or without hormonal contraceptives, that is implanted by a practitioner for the purpose of preventing pregnancy, including intrauterine and implantable devices
Pharmacy Technician and Intern Involvement
Pharmacy technicians who have completed an approved training course for prescribing of hormonal contraceptives may, at the pharmacist's request, assist the pharmacist in prescribing a hormonal contraceptive by:
- providing the screening document to the patient
- taking and recording the patient's blood pressure
- documenting the patient's medical history
A registered pharmacist intern may prepare a prescription for a hormonal contraceptive under the direct supervision of a trained prescribing pharmacist, but a pharmacist authorized to prescribe under this protocol must review, approve, and sign the prescription before the prescription is processed or dispensed.
CONCLUSION
Pharmacist prescribing removes significant barriers to patient access and use of hormonal contraceptives and EC to prevent pregnancy especially for those with limited access to healthcare services or busy schedules. More than 90% of Americans live within five miles of a pharmacy, making pharmacists the most accessible healthcare professionals and perfectly positioned to improve contraceptive access.22 Pharmacist involvement can lead to better education and counseling on contraceptive options, promoting informed decision-making and improving therapy uptake and adherence.
APPENDIX A – CDC US Medical Eligibility Criteria for Contraceptive Use, 2016
APPENDIX B – CDC US Medical Eligibility Criteria for Contraceptive Use, 2016, SUMMARY CHART
APPENDIX A-U.S. MEC 2024_UPDATE full text pdf
 Loading...
Loading...
APPENDIX B-U.S. MEC 2024 Summary Chart
 Loading...
Loading...
Pharmacist Post Test (for viewing only)
Pharmacist Post-test
1. What is the rate of unintended (mistimed or unwanted at the time of conception) pregnancy in the United States?
A. It's about 30%
B. It's about 50%
C. It's about 65%
2. Compared to intentional pregnancy, which of the following conditions is more likely to occur with unintentional pregnancy?
A. Women who experience unintended pregnancy also experience more mental health problems.
B. Women who experience unintended pregnancy also experience higher rates of iron deficiency.
C. Women who experience unintended pregnancy are more likely to be married or in stable relationships.
3. When researchers looked at children born as the result of an unintended pregnancy, what did they find?
A. Their mental and physical challenges are similar to other children's.
B. They are more likely to have mental challenges than physical challenges.
C. They are more likely than other children to struggle in school.
4. Which of the following approaches to contraception prevents implantation of a fertilized ovum in the endometrium?
A. Using a barrier method of contraception
B. Creating an unfavorable uterine environment
C. Preventing ovulation from occurring
5. Hormonal contraceptives use two hormones to prevent pregnancy. What are they?
A. Testosterone and estrogens
B. Progestins and testosterone
C. Progestins and estrogens
6. Which of the following correctly identifies estrogen’s role in birth control?
A. Promote the LH surge, which inhibits ovulation
B. Stabilize the endometrial lining and provide cycle control
C. Increase endometrial thickness to delay implantation
7. Which of the following correctly identifies progestin’s role in birth control?
A. Block the LH surge, which inhibits ovulation
B. Stabilize the endometrial lining and provide cycle control
C. Increase endometrial thickness to delay implementation
8. Why is estrogen never used alone as a contraceptive?
A. This is a trick question. Several FDA approved contraceptives employ estrogen alone to prevent contraception.
B. Unopposed estrogen increases the risk of hormonal imbalance and subsequently, the risk of pregnancy.
C. Unopposed estrogen increases risk of cancer, endometrial hyperplasia, polyps, endometriosis, and adenomyosis in women who have an intact uterus.
9. Anne Marie is 42-year-old woman who comes to the pharmacy seeking hormonal contraception. Your technician takes her blood pressure and records it as 152/93. Anne Marie smells like she has recently smoked a cigarette. Which of the following would be an appropriate choice of contraceptives?
A. A progestin only contraceptive
B. A combined hormonal contraceptive
C. Any hormonal contraceptive would be inappropriate
10. A primary care provider calls the pharmacy and asks you which progestin is the best in terms of contraceptive efficacy. What do you say?
A. No evidence indicates that a particular progestin is superior to others
B. Some evidence indicates any oral progestin is better than injectable forms
C. Norgestimate is more effective than any other progestin
11. When discussing possible hormonal contraceptives, the patient asks about the efficacy of combined oral contraceptives. What do you tell her?
A. With typical use, COCs are about 91% effective, meaning that 9 of 100 women will become pregnant in a year with typical use.
B. With typical use, COCs are about 95% effective, meaning that 5 of 100 women will become pregnant in a year with typical use.
C. With typical use, COCs are about 99% effective, meaning that 1 of 100 women will become pregnant in a year with typical use.
12. You are considering a combined hormonal contraceptive for a patient whose last menstrual period started seven days ago. She will start taking the contraceptive today. What should you tell her about intercourse?
A. She needs no additional protection and can have unprotected intercourse.
B. She should abstain or use backup contraception until her next menses.
C. She should abstain or use backup contraception for the next seven days.
13. Which of the following contains 84 days of active hormone tablets followed by 7 days of inactive tablets?
A. Extended- and continuous-cycle COCs
B. Extended- and continuous-cycle POPs
C. No hormonal contraception is formulated like this
14. A patient expresses a preference for a combined oral contraceptive pill that contains 40 mcg of ethinyl estradiol daily because her friend takes such a pill. She is new to hormonal contraceptives. What do you tell her?
A. You should start a COC containing 35 mcg or less of ethinyl estradiol.
B. You should start a COC containing 20 mcg or less of ethinyl estradiol.
C. You should start a COC containing 50 mcg or more of ethinyl estradiol.
15. The patient in the previous question asks why you selected the answer you did. What do you say?
A. Estradiol levels affect the incidence of blood clotting.
B. Estradiol levels affect the incidence of migraine headache.
C. Estradiol levels affect the incidence of breakthrough bleeding.
16. Why do the guidelines prefer monophasic COCs over multiphasic COCs when women start contraception?
A. Adverse effects are considerably less likely to occur.
B. Monophasic COCs always contain low estradiol doses.
C. Adverse effects are easier to identify and manage.
17. After discussing various options with a patient, she mentions that she works swing shifts in the same week and will sometimes have to take her pill before her night shift and sometimes after her evening shift. She asks if that will be a problem if she prefers the “minipill.” What is the MOST APPROPRIATE question for you to ask?
A. Will that keep you from taking it in the same 3-hour window every day?
B. How long does it take you to drive to and from work?
C. What about the “minipill” do you find so attractive?
18. In which populations of women are progestin-only pills preferred?
A. Postpartum women who have delivered in the last 30 to 42 days and breastfeeding women
B. Women who are older than 42 and smokers and women with body weights less than 127 lbs
C. Women who experience breakthrough bleeding and those with adherence challenges
20. Why do vaginal ring systems carry a risk of toxic shock syndrome?
A. They can trap bacteria on the cervix, allowing it to enter the fallopian tubes via the uterus.
B. The patient handles them multiple times during use, posing a risk of bacterial contamination.
C. They can trap bacteria in the vagina or create tiny cuts, allowing bacteria to enter the uterus via the cervix.
21. What warning is included in depo-medroxyprogesterone acetate’s labeling as a Boxed Warning?
A. Patients should not use the drug for more than two years due to bone mineral density loss, which may be irreversible
B. Return to fertility may be delayed six to 12 months after discontinuation, so DMPA is not recommended for women desiring pregnancy in the near future
C. DMPA is about 89% effective with typical use so women should use a backup method of contraception.
22. Which of the following emergency contraceptives requires a prescription?
A. Levonorgestrel 1.5 mg
B. Ulipristal acetate 30 mg
C. Neither
23. The CDC’s MEC uses “categories” based on evidence to describe its recommendation. Which of the following is paired correctly?
A. 1 = Theoretical or proven risks usually outweigh the advantages
B. 2 = Advantages generally outweigh theoretical or proven risks
C. 4 = No restriction (method can be used)
24. Your patient has sickle cell disease. According to the CDC’s MEC, what category do POPs fall into?
A. 1
B. 2
C. 3
25. Your patient has a history of gallbladder disease and had a cholecystectomy six months ago. Which of the following contraceptive methods are considered appropriate for this patient?
A. Any IUD, implants, or CHCs
B. DMPA, POPs, and CHCs
C. Benefits generally outweigh the risks for all methods
26. You look at the CDC’s MEC summary chart and you find that the category you are considering is marked with an asterisk (*). What does that mean?
A. Condition that exposes a woman to increased risk as a result of pregnancy.
B. See the complete guidance for a clarification to this classification.
C. Advantages generally outweigh theoretical or proven risks.
27. You look at the CDC’s MEC summary chart and you find that the category you are considering is marked with a dagger (‡). What does that mean?
A. Condition that exposes a woman to increased risk as a result of pregnancy.
B. See the complete guidance for a clarification to this classification.
C. Advantages generally outweigh theoretical or proven risks.
28. In the CDC’s MEC, what is the daily threshold for number of cigarettes at which the risk of using a CHC increases to “Unacceptable health risk (method not to be used)” for women age 35 and older?
A. 15
B. 20
C. 30
29. What does the CDC’s MEC say about the evidence to support the use of progestin-only injectable contraceptives in women at high risk for HIV?
A. Eleven observational studies suggested no association between their use and HIV acquisition
B. Eleven observational studies suggested their use increased risk for HIV acquisition 3-fold
C. Insufficient evidence exists to conclude that they increase the risk of HIV acquisition
30. In a woman who has rheumatoid arthritis and takes immunosuppressives, which of the following poses the highest risk?
A. DMPA
B. POP
C. CHC
31. What is the U.S. MEC’s reason for classifying the class of drugs referred to in the previous question as they did?
A. Risk for breakthrough bleeding increases
B. Risk for osteoporosis increases
C. Risk for drug interactions increases
32. What does the U.S. MEC, indicate about the use of emergency contraceptive pills (ECPs) in women who have experienced sexual assault?
A. ECPs might be less effective among women with BMI < 25 kg/m2 than among women with BMI > 30 kg/m2.
B. Frequently repeated ECP use might be harmful for women with conditions classified as 1, 2, or 3 for CHC or POP use.
C. Women with obesity might experience an increased risk for pregnancy after use of ulipristal acetate compared with women of healthy weight.
33. Carmen is a 44-year-old mother of four. She recently experienced a pulmonary embolism. Which hormonal contraceptive is LEAST appropriate for her?
A. Depo-medroxyprogesterone acetate
B. Combined hormonal contraceptives
C. Progestin-only pills
34. Alexis is on a COC and is experiencing early cycle (days 1-9) breakthrough bleeding. What change to her COC might resolve this issue?
A. Using a COC with more progestin
B. Using a COC with more estrogen
C. Changing her to a POP
35. Justine has been using DMPA for two years, and really likes it for its convenience. She asks you to renew the prescription. What do you do?
A. Screen her for adverse effects and renew the prescription if she has none.
B. Renew the prescription and advise her to increase her calcium and vitamin D intake.
C. Explain why it’s necessary to find an alternative birth control at this point.
36. Rory comes to the pharmacy requesting a prescription for emergency contraception following unprotected sex 48 hours ago. She weighs 150 lbs and has missed the first three doses of her COC because she forgot to refill it on time. Which of the following is the BEST choice for Rory?
A. Prescribe levonorgestrel
B. Prescribe ulipristal acetate
C. Referral for an emergency IUD
37. Tina is a 27-year-old woman who is six months postpartum and requesting a prescription for hormonal contraceptives. Her baby is formula-fed, she weighs 205 lbs, and she takes sertraline (an SSRI) for postpartum depression. She explains that she has had trouble with daily medication adherence in the past and expresses concerns about intolerable adverse effects. Which of the following hormonal contraceptives is MOST appropriate for Tina?
A. Continuous-cycle COCs
B. Transdermal CHC patch
C. EE/E vaginal ring system
38. In addition to verbal counseling, what must a pharmacist provide when prescribing hormonal contraception?
A. A document describing storage
B. A fact sheet specific to the drug
C. A receipt indicating the drug’s cost
39. In addition to the document described in the previous question, what else should the patient have in her possession before leaving the pharmacy?
A. A copy of documentation that the pharmacist passed this test
B. The pharmacist’s business card that includes a phone number
C. A written record of the contraceptive prescribed
40. Which of the following is a critical counseling point when counseling a woman who is starting hormonal contraception after using ECP?
A. Hormonal contraceptives do not protect against sexually transmitted diseases.
B. Using hormonal contraceptives routinely is less expensive than using ECPs.
C. Once you start hormonal contraception, you cannot use ECPs again.
41. You are prescribing POPs to a patient who started her last menstrual period 10 days ago. The last time she had sexual intercourse was 14 days ago. Which of the following counseling points is appropriate?
A. Start this medication today, and no backup contraception is needed.
B. Start this medication today and use backup contraception for 2 days.
C. Do not start this medication until after you’ve taken a pregnancy test.
42. You are prescribing transdermal CHCs to a patient who was previously on COCs but had poor adherence. Which of the following counseling points is appropriate?
A. If the patch detaches for three or more hours, use backup contraception for seven days
B. If you plan to exercise, use medical tape to reinforce the patch and prevent detachment
C. You may experience more adverse effects due to higher estrogen exposure
43. A patient consults with you for emergency contraception. You decide that ulipristal acetate is the best choice. The patient asks you to give her four refills. What do you tell her?
A. Yes, I can give you four refills but you must use them within a year.
B. Ulipristal is an OTC drug; you don’t need a prescription or refills.
C. The law doesn’t allow refills, so let’s discuss a better contraceptive plan.
44. Your patient is interested in hormonal contraception. She indicates that she doesn’t remember when she last saw a healthcare provider for a women’s health visit. Under what circumstance can you prescribe hormonal contraception?
A. She has an empty package of oral contraceptives and the last refill was expended this month.
B. She is younger than 18 years of age and accompanied by her mother who says it’s OK to prescribe to her.
C. She has a package of ECPs prescribed by a pharmacist in another state about two and a half years ago.
45. It’s August and a college student visits and asks for a prescription for a hormonal contraceptive. She’s excited because she is going to study abroad for one year starting in October. She asks for 14 months of an OCP. What do you do?
A. Write a prescription with 14 one-month refills and tell her to have her parent refill it and mail it as necessary
B. Issue a prescription for three months, and ask her to return before she leaves for a new 1-year prescription
C. Fill it in bulk for 14 months and make a note in the record indicating this is an exception to policy allowed by law
46. A 16-year-old high school student asks for a prescription for a hormonal contraceptive. In what way is this situation different than handling a prescription for a patient older than 18?
A. The patient must have previously been prescribed a contraceptive by another provider.
B. The patient needs permission from a parent or guardian before a pharmacist can prescribe.
C. The patient must take an OTC pregnancy test before a pharmacist can prescribe.
47. A woman comes to the pharmacy with a prescription for hormonal contraception written by another pharmacist. Can you fill it?
A. Yes, if I know the pharmacist.
B. No, she needs to go back to the pharmacy where the prescribing pharmacist works.
C. Yes, if the prescribing pharmacist is authorized to write for hormonal contraception.
48. Which of the following is a red flag requiring pharmacist referral to a practitioner for evaluation before prescribing DMPA?
A. Blood pressure > 140/90 mmHg
B. Taking escitalopram for depression
C. Gave birth 3 weeks ago
49. After prescribing hormonal contraceptives, which of the following is required?
A. Maintain completed screening documents at the prescribing pharmacy for 1 year
B. Transmit dispensing information to the electronic prescription drug monitoring program within 72 hours
C. Maintain all records created as part of the prescribing process for three years
50. Your pharmacy is located in a college town, and you are inundated with requests for hormonal contraceptive prescriptions. One of the pharmacy technicians completed an approved training course and would like to help. Which of the following is an appropriate way for the technician to contribute?
A. Provide screening documents and take and record the patient’s blood pressure
B. Take and record the patient’s blood pressure and review screening documents for contraindications
C. Supervise patients performing OTC pregnancy tests and provide educational materials about contraceptives
References
Full List of References
REFERENCES
- Guttmacher Institute. Contraceptive use in the United States by demographics. May 2021. Accessed October 18, 2023. https://www.guttmacher.org/fact-sheet/contraceptive-use-united-states
- Office on Women’s Health. Unplanned pregnancy. Updated February 22, 2021. Accessed October 18, 2023. https://www.womenshealth.gov/pregnancy/you-get-pregnant/unplanned-pregnancy
- Office of Disease Prevention and Health Promotion. Healthy People 2030: Reduce the proportion of unintended pregnancies — FP‑01. Accessed October 18, 2023. https://health.gov/healthypeople/objectives-and-data/browse-objectives/family-planning/reduce-proportion-unintended-pregnancies-fp-01
- Centers for Disease Control and Prevention. Contraception. Updated May 1, 2023. Accessed October 18, 2023. https://www.cdc.gov/reproductivehealth/contraception/index.htm
- Bull JR, Rowland SP, Scherwitzl EB, Scherwitzl R, Danielsson KG, Harper J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit Med. 2019;2:83. doi:10.1038/s41746-019-0152-7
- Reed BG, Carr BR. The normal menstrual cycle and the control of ovulation. In: Feingold KR, Anawalt B, Blackman MR, et al., eds. Endotext. South Dartmouth (MA): MDText.com, Inc.; August 5, 2018.
- Teal S, Edelman A. Contraception selection, effectiveness, and adverse effects: A Review. JAMA. 2021;326(24):2507-2518. doi:10.1001/jama.2021.21392
- Montanino Oliva M, Gambioli R, Forte G, Porcaro G, Aragona C, Unfer V. Unopposed estrogens: current and future perspectives. Eur Rev Med Pharmacol Sci. 2022;26(8):2975-2989. doi:10.26355/eurrev_202204_28629
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65(4):1-66. doi:10.15585/mmwr.rr6504a1
- Annovera (prescribing information). TherapeuticsMD, Inc.;2022.
- Centers for Disease Control and Prevention. Recommended Actions After Late or Missed Combined Oral Contraceptives. Accessed February 28, 2024. https://www.cdc.gov/reproductivehealth/contraception/pdf/recommended-actions-late-missed_508tagged.pdf
- American College of Obstetricians and Gynecologists. Effectiveness of birth control methods. April 2023. Accessed February 20, 2024. https://www.acog.org/womens-health/infographics/effectiveness-of-birth-control-methods
- Darney PD. OC practice guidelines: minimizing side effects. Int J Fertil Womens Med. 1997;Suppl 1:158-169.
- Ortho Evra (prescribing information). Janssen Pharmaceuticals, Inc.;2017.
- Annovera - a new contraceptive vaginal ring. Med Lett Drugs Ther. 2019;61(1587):197-198.
- NuvaRing (prescribing information). Organon & Co.; 2022.
- Cleveland Clinic. Toxic shock syndrome. Updated August 12, 2022. Accessed February 20, 2024. https://my.clevelandclinic.org/health/diseases/15437-toxic-shock-syndrome
- Depo-subQ Provera 104 (prescribing information). Pfizer Inc.; 2020.
- Plan B One-Step (prescribing information). Barr Laboratories; 2009. https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021998lbl.pdf
- Ella (prescribing information). HRA Pharma America Inc.; 2021.
- Centers for Disease Control and Prevention. US Medical Eligibility Criteria for Contraceptive Use, 2016 (US MEC). Reviewed March 27, 2023. Accessed February 20, 2024. https://www.cdc.gov/reproductivehealth/contraception/mmwr/mec/summary.html22. National Association of Chain Drug Stores. Pharmacies - The Face of Neighborhood Health Care Since Well Before the Pandemic. Accessed March 1, 2024. https://www.nacds.org/pdfs/about/rximpact-leavebehind.pdf