Learning Objectives
After completing this application-based continuing education activity, pharmacists will be able to
- Discuss current theories postulating how Sjogren’s syndrome develops
- Identify biomarkers used in diagnosis and patient classification
- Interpret guidelines and evidence-based medicine to use best practices to manage Sjogren’s syndrome
- Use elements of an integrated approach to care among specialists and other pharmacists
After completing this application-based continuing education activity, pharmacy technicians will be able to
- Describe Sjogren’s syndrome’s basic pathology and symptoms
- Outline prescription and non-prescription treatments used in Sjogren’s syndrome
- Identify when to refer patients to the pharmacists for recommendations or referrals

Release Date:
Release Date: July 1, 2022
Expiration Date: July 1, 2025
Course Fee
FREE
An Educational Grant has been provided by:
Novartis
ACPE UANs
Pharmacist: 0009-0000-22-047-H01-P
Pharmacy Technician: 0009-0000-22-047-H01-T
Session Codes
Pharmacist: 22YC47-FKW24
Pharmacy Technician: 22YC47-WKW44
Accreditation Hours
2.0 hours of CE
Accreditation Statements
| The University of Connecticut School of Pharmacy is accredited by the Accreditation Council for Pharmacy Education as a provider of continuing pharmacy education. Statements of credit for the online activity ACPE UAN 0009-0000-22-047-H01-P/T will be awarded when the post test and evaluation have been completed and passed with a 70% or better. Your CE credits will be uploaded to your CPE monitor profile within 2 weeks of completion of the program. |  |
Disclosure of Discussions of Off-label and Investigational Drug Use
The material presented here does not necessarily reflect the views of The University of Connecticut School of Pharmacy or its co-sponsor affiliates. These materials may discuss uses and dosages for therapeutic products, processes, procedures and inferred diagnoses that have not been approved by the United States Food and Drug Administration. A qualified health care professional should be consulted before using any therapeutic product discussed. All readers and continuing education participants should verify all information and data before treating patients or employing any therapies described in this continuing education activity.
Faculty
Kelsey Giara, PharmD
Freelance Medical Writer
Pelham, NH
Faculty Disclosure
In accordance with the Accreditation Council for Pharmacy Education (ACPE) Criteria for Quality and Interpretive Guidelines, The University of Connecticut School of Pharmacy requires that faculty disclose any relationship that the faculty may have with commercial entities whose products or services may be mentioned in the activity.
Dr. Giara has no relationship with ineligible companies and therefore has nothing to disclose.
ABSTRACT
Once considered a “dry eye-dry mouth-arthritis” illness, Sjogren’s syndrome (SjS) is a systemic condition strongly associated with organ-specific and systemic autoimmunity. Systemic SjS is linked to autoimmune dysfunction that may eventually be irreversible. This disease affects about 2 to 4 million Americans, but every patient presents differently and symptoms mimic those of various other conditions, posing a challenge for diagnosis and treatment. SjS’s classic symptoms include sicca (ocular and oral dryness), arthralgia (joint pain), and fatigue. The pathogenesis of the disease is complex and multifactorial, but researchers are looking for a well-defined cause and modern understanding of SjS is improving. The search for biomarkers, therapeutic targets, and disease-modifying treatments for SjS is underway. Health care providers—including pharmacists and pharmacy technicians—who are up to date on current understanding and recently-updated guidelines will be better prepared to make evidence-based recommendations and appropriate referrals to improve care and outcomes for patients with SjS.
CONTENT
Content
INTRODUCTION
The medical community’s understanding of Sjogren’s syndrome (SjS) has evolved a great deal since it was first recognized in the late 1800s. A surgeon reported the first clinical case of what is now called SjS in 1888, describing a male patient with painless bilateral swelling of the lacrimal, parotid, and submandibular glands (i.e., the glands that produce tears and saliva).1 Following a series of case reports over about a century detailing a “dry eye-dry mouth-arthritis” illness, physicians pieced together and named the syndrome known today as SjS.1,2
Epidemiologic data about SjS in the United States (U.S.) is limited. It is estimated to affect about 2 to 4 million Americans, but only about 1 million are definitively diagnosed.3,4 Women are nine times more likely to have the condition, and it typically emerges around menopause (i.e., after age 50). SjS is the second most common rheumatologic disorder in the U.S. behind systemic lupus erythematous (SLE).5 Autoimmune conditions don’t discriminate; many famous people have historically battled them publicly. Selena Gomez postponed a concert tour to undergo treatment for SLE. Kim Kardashian suffers from psoriasis. SjS, as a rarer condition, doesn’t make the news quite as often as other autoimmune conditions, but here are a few people you may recognize who are battling the disease today6-8:
- Carrie Ann Inaba: In 2021, the 30-season judge of Dancing with the Stars and co-host of The Talk took a leave of absence from television to focus on her health and wellbeing. The chronic pain associated with her SjS, SLE, fibromyalgia, and rheumatoid arthritis forced her to stay in bed three days a week.
- Shannon Boxx: This World Cup soccer player and Olympic gold medalist was diagnosed with SjS in 2002 and suffered from severe fatigue and joint pain. Ahead of the 2007 World Cup, she was put on corticosteroids to alleviate her symptoms and needed specific approval from the U.S. Anti-Doping Agency to take them while competing.
- Venus Williams: While dominating the sport of tennis as the most decorated female tennis player to compete in the Olympic Games, she has also been in a battle against her own body. SjS-related fatigue caused her to pull out of the 2011 U.S. Open, and she was temporarily booted from the top 100 tennis players for the first time in 15 years.
As an autoimmune condition, SjS’s cause is unclear. Genetic, environmental, and hormonal factors likely work collaboratively to produce the cardinal symptoms of dry eyes and/or mouth, fatigue, and limb pain. Some patients experience additional manifestations in the lymph nodes, lungs, kidneys, muscles, nervous system, skin, teeth, and brain. Glandular and joint involvement is also possible, and constitutional symptoms (e.g., fever, involuntary weight loss, night sweats) can affect quality of life. Patients with SjS have an elevated risk of lymphoma, about 15 to 20 times higher than the general population.9,10
PAUSE AND PONDER: Why are patients with SjS so difficult to identify and diagnose?
Clinical Presentation
SjS is a systemic condition strongly associated with organ-specific and systemic autoimmunity. Since it impacts multiple systems in the body, SjS can manifest in various ways. Affected patients may have symptoms that cycle between mild and severe. Symptoms also tend to worsen as patients age and the function of the exocrine glands subsides.
SjS’s main symptoms are dry mouth and dry eyes (collectively, sicca). More than 95% of patients with SjS present with sicca symptoms, which are irritating and poorly tolerated.11 About half of patients also have dermatologic involvement (i.e., dry skin or rashes).3,12 Xerostomia (oral dryness) can substantially impact daily life, interfering with eating, speaking, or sleeping.4 When patients’ salivary volume decreases, they also lose saliva’s antibacterial properties. This can accelerate tooth decay, infection, and periodontal disease. Patients with dry mouth also report swallowing difficulties, halitosis (bad breath), and burning sensations in the mouth. Using artificial saliva products to manage dry mouth is time-consuming and ineffective for many patients with SjS.13
Patients with ocular dryness complain of itchy, gritty, sore, or dry sensations in the eyes despite appearing physically normal.4 Decreased tear production over time can cause chronic irritation and destruction of conjunctival epithelium that lines the inside of the eyelids and covers the sclera (whites of the eyes).
Patients may also experience symptoms elsewhere in the body, including
- Dry cough
- Fatigue
- Joint and muscle pain
- Numbness or tingling of the hands and feet
- Vaginal dryness
Patients who develop musculoskeletal symptoms may have difficulty remaining active. About 53% of patients experience arthralgias (joint stiffness) and 22% experience myalgias (muscle pain).4 SjS-associated arthralgia occurs primarily in small joints, sometimes asymmetrically. Providers may confuse these symptoms with SLE or rheumatoid arthritis.
Disease Classification and Severity
Experts classify SjS as primary or secondary (see Table 1). Primary SjS (pSjS) is an autoimmune disease that causes immune cells to mistakenly attack and destroy healthy cells in the glands that produce tears and saliva. SjS can also be secondary to other autoimmune diseases (e.g., SLE, rheumatoid arthritis, scleroderma), as is the case for about 60% of patients.4
Table 1. American-European Consensus Group Criteria for the Classification of SjS14
| Primary SjS Criteria | Secondary SjS Criteria | SjS Exclusion Criteria |
| At least 4 of the following, including at least criterion 5 or 6:
1. Ocular symptoms (dry eyes for ≥ 3 months, foreign-body sensation, use of tear substitutes > 3 times daily) 2. Oral symptoms (dry mouth, recurrently swollen salivary glands, frequent use of liquids to aid swallowing) 3. Ocular signs (Schirmer test* performed without anesthesia [< 5 mm in 5 minutes], positive vital dye staining results) 4. Oral signs (abnormal imaging of salivary glands, unstimulated salivary flow < 1.5 mL in 15 minutes) 5. Positive minor salivary gland biopsy findings 6. Positive anti-SSA or anti-SSB antibody results |
In the presence of a connective-tissue disease, symptoms of oral or ocular dryness exist in addition to criterion 3, 4, or 5 for primary SjS. | Any of the following:
· AIDS · Graft versus host disease · Hepatitis C virus infection · Past head-and-neck radiation · Prior lymphoma · Sarcoidosis · Use of anticholinergic drugs |
*Schirmer test is used to determine whether the eye produces enough tears to keep it moist; AIDS = acquired immunodeficiency syndrome; SjS = Sjogren’s syndrome; SSA = Sjogren's syndrome A; SSB = Sjogren’s syndrome B
There is a broad range of disease severity in SjS. Some patients experience mild glandular dryness and constitutional symptoms while others have severe glandular involvement and various manifestations throughout the rest of the body, including systemic autoimmune features. Mild SjS has a good prognosis, but patients often have difficulty managing their symptoms and moderate-to-severe disease can severely impact quality of life.15 SjS symptoms cause considerable psychological distress. About one third of patients with the condition have clinically significant anxiety and half have diagnosable depression.16
Measuring Systemic Disease Activity
The European League Against Rheumatism (EULAR) created a disease activity index for primary SjS (ESSDAI) to measure systemic disease activity.17,18 The ESSDAI includes 12 domains: cutaneous, respiratory, renal, articular, muscular, peripheral nervous system, central nervous system, hematological, glandular, constitutional, lymphadenopathic, and biological. Each domain is divided into three or four levels of activity, and patients are scored based on that domain’s severity (i.e., 0 indicates no activity and 3 or 4 indicates high activity).18
Each domain’s weight reflects its relative importance to disease activity, and the score for each domain is equal to the level of activity multiplied by the domain’s weight. A final ESSDAI score (i.e., the sum of all the domain scores) could theoretically be between 0 and 123. Patients’ disease activity based on ESSDAI score is as follows17,18:
- 0 = no activity
- 1 to 4 = low activity
- 5 to 13 = moderate activity
- 14 or greater, or high activity in any domain with a definition of high activity = severe activity
Measuring Patient-Reported Outcomes
EULAR also created the SjS patient reported index (ESSPRI) to assess patient-reported outcomes in pSjS.18 This scale focuses solely on the three major manifestations of SjS: dryness, fatigue, and musculoskeletal pain. Patients rank each of these domains on a scale of 0 to 10, and the total ESSPRI score is the mean (average) of those scores. A “patient acceptable symptom state” is defined as an ESSPRI score of less than 5, and clinicians and researchers define “minimally clinically important improvement” as an increase in ESSPRI score 1 point or more or 15%.18
PAUSE AND PONDER: How would your daily life change if you had SjS? What hardships might you face?
Recognition and Treatment are Inadequate
SjS’s variable symptoms are not always present at the same time, leading providers—including physicians, dentists, and ophthalmologists—to treat each symptom individually, unaware of the systemic disease’s presence. Patients suffer from SjS symptoms an average of 10 years before obtaining a diagnosis.4,19 The condition has historically been misdiagnosed because providers consider symptoms minor or vague and they often mimic other diseases. Up to 30% of people 65 years or older, with SjS or not, report dryness of the eyes and mouth.19 Sicca and/or parotid gland enlargement can result from various other conditions, including19
- Alzheimer’s disease
- anxiety and depression
- Bell’s palsy
- bulimia
- chronic conjunctivitis or blepharitis (inflammation of the membrane on the eye or the eyelid, respectively)
- chronic pancreatitis
- complications from contact lenses
- dehydration
- diabetes mellitus
- hepatitis C
- Parkinson’s disease
- rosacea
- viral infections (e.g., cytomegalovirus, influenza, mumps)
About half of patients with SjS lack a definitive diagnosis, so undertreatment is considerable.4 For those who are diagnosed, treatment guidelines have historically been unclear and available treatments are limited and often unsuccessful. Recently, evidence-based treatment guidelines have emerged (discussed below) to help providers make decisions regarding SjS care. SjS is incurable; targeted, disease-modifying therapies are needed.
DISEASE MECHANISMS AND BIOMARKERS
pSjS’s pathogenesis is complex and multifactorial. Underlying genetic predisposition, epigenetic mechanisms (i.e., things that cause changes that affect the way your genes work), and environmental factors contribute to disease development.20 There is no identified causal agent for SjS and it presents with multiple organ involvement. This makes the pursuit for defining an etiology and identifying biomarkers all the more important.
Researchers historically considered SjS a specific, self-perpetuating immune-mediated loss of exocrine tissue as the main cause of glandular dysfunction.20 Today, with more sophisticated research methods, experts believe this fails to fully explain several SjS-related phenomena and experimental findings.
Genetics and Epigenetics
Genetic studies are a powerful tool for discovering new pathogenic pathways. Scientists have made great strides in studying genetic susceptibility to pSjS, but the evidence still does not match that of other autoimmune conditions.20 Several genome-wide association studies in pSjS have shown that the strongest association lies within human leukocyte antigen (HLA) genes.
The non-HLA genes IRF5 and STAT4 (relevant to the innate and adaptive immune systems) also show consistent associations but on a smaller scale.20 These genes activate interferon (IFN) pathways as part of the innate immune system. Epigenetic mechanisms (e.g., DNA methylation) also play a role in pSjS pathogenesis by modulating gene expression without altering DNA sequences. This may serve as a dynamic link between the genome and SjS manifestation.
Chronic Immune System Activation
Chronic immune system activation is central to SjS pathophysiology. Innate (“nonspecific”) immunity is the defense system people are born with to protect them from all antigens (foreign substances) that enter the body. Unlike the innate immune system, which attacks based on identification of general threats, the adaptive immune system is activated by pathogen exposure. Adaptive immunity uses its “memory” to learn about the threat and enhance the immune system accordingly over time. The adaptive immune system relies on B cells and T cells—otherwise known as lymphocytes—to function.
IFNs exert antiviral, antimicrobial, antitumor, and immunomodulatory effects as part of the innate immune system. Literature widely recognizes the SjS-associated “IFN signature,” as increased IFN levels activate multiple IFN-responsive genes involved in immune activities.21 Research indicates that type 1 IFN dysregulation is a major pathogenic mechanism in many autoimmune conditions, including SjS.21,22 It is also suggested that “crosstalk” between the type 1 IFN pathway and B-cell activation causes a vicious cycle of immune activation where type 1 IFNs drive production of autoantibodies (made against substances formed by a person's own body) which further promotes IFN production.21 Toll-like receptors (TLRs) also play key roles in the innate immune system.21 Research suggests that TLR-dependent IFN expression may contribute to immune system activation and autoimmunity development in pSjS.21
In patients with SjS, lymphocytes infiltrate the salivary and lacrimal glands and other glands of the respiratory and gastrointestinal tracts and vagina.4 T cells in this infiltrate produce interleukin (IL)-2, IL-4, IL-6, IL-1β, and tumor necrosis factor while the B cells cause hypergammaglobulinemia (overproduction of immunoglobulins/antibodies) and produce autoantibodies.4 Some of these autoantibodies target cellular antigens of salivary ducts, the thyroid gland, the gastric mucosa, erythrocytes, the pancreas, the prostate, and nerve cells. About 60% of patients with SjS also express non-organ-specific autoantibodies, including rheumatoid factors, antinuclear antibodies, and antibodies to the small RNA-protein complexes Ro/SS-A and La/SS-B.4 These processes eventually lead to glandular dysfunction that manifests as dry eyes and mouth and enlargement of major salivary glands.
B-cell activating factor (BAFF) may also contribute to pSjS development. BAFF is usually an active part of the innate immune system, but B cells, T cells, and epithelial cells in the salivary glands also release BAFF in response to IFNs.21 This suggests that epithelial cells are not only passive victims of pSjS autoimmunity but also contributors to immune system overactivation. This also shows that BAFF serves as a link between the innate and adaptive immune systems in pSjS and could also represent an important therapeutic target in pSjS.21
Other Theories
Research into a well-defined cause of SjS is ongoing. Additional theories include a potential viral trigger, neuroendocrine abnormalities, and autoimmune epithelitis. Evidence for a viral trigger in pSjS development is conflicting, but studies have been unable to replicate an association between SjS and Epstein-Barr virus, hepatitis C virus, retroviruses, or Coxsackie A virus.21 Researchers think that the microbial stimuli driving pSjS development could be diverse or that the initiating viral stimulus is no longer detectable once the disease manifests.
The classic triad of symptoms in pSjS is sicca, arthralgia, and fatigue. Pathogenic mechanisms producing fatigue remain unknown, but neuroendocrine dysfunction may play a key role in the process.21 Studies show that patients with pSjS have decreased hormone levels (e.g., cortisol) compared with healthy individuals, indicating dysfunction of the hypothalamic-pituitary-adrenal (HPA) axis. The HPA axis is suggested to promote fatigue and depression in patients with pSjS.
The Search for Biomarkers
Disclosing a disease’s etiology allows researchers to identify biomarkers (i.e., a biological molecule in the body that is a sign of an abnormal process) for diagnosis and assessment of disease process and treatment response. It also stimulates discovery of treatment targets. Researchers have been hard at work searching for biomarkers for SjS. Biomarkers can drive more precise diagnosis and may be used to measure disease severity or see how well the body responds to treatment. Scientists have discovered potential biomarkers, but studies have yet to validate their utility in SjS diagnosis and treatment.
Novel tissue-specific autoantibodies (TSAs) have been described in the early stages of pSjS, including salivary protein-1, parotid secretory protein, and carbonic anhydrase 6.23 These are detectable even before the classic autoantibodies Ro/SS-A and La/SS-B. Further studies are needed to determine the utility of TSAs in screening patients with dry eye for SjS.
Some researchers hope to look beyond blood for reliable biomarkers for pSjS, more specifically in tears or saliva. They have studied tear proteins LACTO and LIPOC-1 as potential biomarkers for pSjS and one study shows they are more accurate indicators than traditional clinical tests for disease detection.23 Other studies have examined salivary levels of S100A8/A9 as a potential biomarker for lymphoma development in patients with pSjS. Imaging biomarkers are also gaining attention. Salivary gland ultrasounds, for example, are non-invasive and valuable for studying the morphology (structure) of major salivary glands.23
EVIDENCE-BASED TREATMENT STRATEGIES
SjS has no cure, and treatment varies from person to person based on their symptoms. Until recently, guidelines were unavailable to help clinicians manage Sjogren’s syndrome rationally.
In 2019, EULAR released evidence-based guidelines on which to rely, but clinicians may be unaware of its availability.11 Medication is the cornerstone of these recommendations, so pharmacists and pharmacy technicians should be prepared to make evidence-based recommendations and appropriate referrals to improve care for patients with SjS. It is important to remember that no therapy is explicitly approved for SjS disease modification. Rather, providers use therapies indicated for each symptom separately—and some off-label (i.e., for a non-FDA-approved indication)—on a trial-and-error basis based on available evidence from small trials that sometimes include a subset of patients with SjS.
Sicca
Glandular dysfunction—the cause of sicca symptoms—appears to be stable for long periods of time (up to 12 years) and has a chronic course in patients with pSjS.11 No therapeutic intervention can reverse or slow the progression of glandular dysfunction, so sicca symptoms cannot be cured. EULAR guidelines state that the first therapeutic approach to sicca symptoms should be symptomatic relief using topical therapies (e.g., saliva substitutes, artificial tears).11 This minimizes the risk of adverse effects (AEs) seen with systemic therapies.
Finding the optimal lubrication is a matter of trial and error, so pharmacy staff should be prepared to set realistic expectations for patients seeking relief of sicca symptoms. They should also help patients recognize when it may be time to talk to their prescriber about stepping up to pharmacologic treatment.
Oral Dryness
Treatment of oral dryness depends on the severity of salivary gland dysfunction (Figure 1).11 No evidence indicates any non-pharmacologic stimulant is better than another, so patients with mild glandular dysfunction should use a trial-and-error approach to find one that works for them. If these therapies don’t help or patients do not wish to use non-pharmacologic stimulants, providers should move on to pharmacologic stimulation. Muscarinic agonists’ main AE is excessive sweating.11 To avert this, EULAR recommends increasing the dose progressively up to 15 to 20 mg/day when possible.
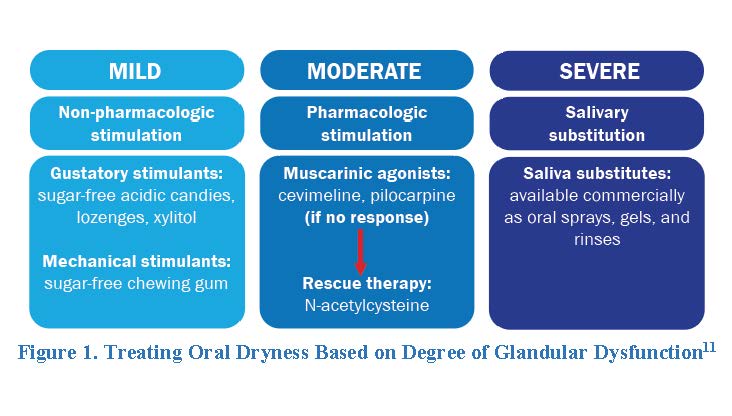
Cevimeline and pilocarpine are cholinergic agents, meaning they activate muscarinic receptors in the parasympathetic nervous system (which controls bodily functions when a person is at rest) to stimulate saliva production. Patients with SjS take cevimeline 30 mg by mouth three times daily or pilocarpine 5 mg by mouth four times daily to treat dry mouth.24,25 The most common AEs of cevimeline and pilocarpine are excessive sweating, nausea, rhinitis (stuffy nose), and diarrhea.
The ideal saliva substitute will have a neutral pH mimicking natural saliva composition and contain fluoride and other electrolytes.11 Gel formulations are ideal for patients with acceptable salivary flow output, especially those with oral dryness at night. However, patients often dislike these formulations due to their sticky mouthfeel. Pharmacists can recommend that patients dilute oral gel formulations to reduce this phenomenon for better adherence. Thinner preparations are preferred for patients with better-preserved glandular function.11
Some experts feel that all patients with oral dryness should use salivary substitutes regardless of the degree of glandular dysfunction.11 Whether patients use these formulations or not, all patients with salivary dysfunction should use a neutral pH sodium fluoride gel to prevent extensive caries (i.e., cavities).
Ocular Dryness
Reflex tears are the tears we produce when we cry, while we produce basal tears continuously to lubricate the ocular surface (the surface layers of the eye, namely the cornea and conjunctiva).26 While tears may taste like salt water, their composition is more complex. Both types of tears contain water, but they also contain mucin, lipids, proteins (lysozyme, lactoferrin, lipocalin, immunoglobulins, and peroxidase), electrolytes (sodium, potassium, chloride, bicarbonate, magnesium, and calcium), growth factors (epidermal growth factor), cytokines, and glucose.
Artificial tears and ocular gels/ointments are first line therapies for volume replacement and lubrication for ocular dryness.11 While many people refer to over-the-counter (OTC) drops, gels, ointments, and lubricants as artificial tears, these products lack the biologically active components found in natural tears.27 Their primary role is to supplement the patient’s natural tear production and provide sufficient lubrication to avoid eye complications.
Many artificial tear formulations are available, so patients may need assistance navigating the options. Table 2 lists common ingredients in artificial tears and their functions. A major difference between OTC products is the presence of chemical formulations that increase viscosity (thickness/stickiness) and adhesion and facilitate even distribution across the ocular surface.27 As a general rule, drops are the lowest viscosity products, ointments have the highest viscosity, and suspensions fall in between. Lubricants with a polymeric base or viscosity agent are preferred for patients with SjS to11
- Add volume to the tear lake
- Increase the time the lubricant remains on the ocular surface
- Cushion the ocular surface to reduce friction between the eye and the eyelid
Table 2. Artificial Tear Ingredients and Their Functions27-30
| Ingredient Class | Function | Examples |
| Astringent | Precipitate protein to clear mucus from outer eye surface | zinc sulfate |
| Buffering agent | Maintain normal tear film pH | bicarbonate, phosphate |
| Demulcent | Protect and lubricate mucous membrane surfaces | carboxymethylcellulose sodium, dextran, gelatin, glycerin, hydroxyethyl cellulose, hypromellose, methylcellulose, polyethylene glycol, polysorbate, polyvinyl alcohol, povidone, propylene glycol |
| Lipid formulations | Improve gland function and increase tear film stability | castor oil, phospholipids, triglycerides |
| Preservatives | Prevent bacterial contamination | benzalkonium chloride (BAK), ethylenediaminetetraacetic acid (EDTA), polyquaternium-1, sodium chlorite, sodium perborate |
Not all artificial tear products are equal, and different products work better for different patients. The optimal artificial tear offers long-lasting, effective symptom relief. It should also have low blur and be comfortable to administer.31 Surface tension, pH, viscosity, duration of action, and preservative presence or absence affect these factors.
OTC eye drops commonly include preservatives to prevent bacterial contamination. Repeated use of preservative-containing eye drops is associated with ocular allergies and toxicities, which can lead to product nonadherence and worsening symptoms.27 Benzalkonium chloride (BAK)—the most common preservative used in eye formulations—is an epitheliotoxin and a toxic detergent.28,29 It attracts monocytes and lymphocytes to the conjunctiva, worsening inflammation and thickening the tissue. This effect is cumulative; the more the eye is exposed to BAK, the greater the negative effects.29 As a rule-of-thumb, pharmacists should always recommend products without BAK as a preservative.28
EULAR recommends that all patients with SjS who present with ocular dryness use artificial tears containing methylcellulose or hyaluronate at least twice daily.11 They should increase frequency as symptoms reappear as often as hourly. Individuals who use artificial tears four or more times daily should always use preservative-free products. Patients who experience overnight dryness should consider ophthalmic ointments before bedtime, as they remain in the eye longer. These are not recommended for daytime use because they blur vision.
Patients who are refractory to artificial tears and ointments—those who do not improve after maximum use—should see an ophthalmologist for prescription treatment. Short-term non-steroidal anti-inflammatory drug (NSAID) or corticosteroid eye drops are indicated for a maximum of two to four weeks.11 This is due to the potential for AEs with long-term use, including
- NSAIDs: corneal-scleral melts, perforation, ulceration, severe keratopathy
- corticosteroids: infections, increased intraocular pressure, cataract worsening or development
Cyclosporine 0.05% is another therapeutic option for patients who are refractory to or do not tolerate artificial tears and ointments and those with severe ocular dryness requiring multiple courses of a glucocorticoid eye drop.11 Cyclosporine is a calcineurin inhibitor that prevents T cell maturation.32 This counteracts SjS’s vicious cycle of inflammation. Patients administer the drug in the eyes twice daily, and the most common AEs are eye burning, stinging, and irritation. Of note, a small trial investigating topical tacrolimus showed promising results, but larger trials are needed to confirm the role of this drug for SjS-associated ocular dryness. Some providers also use lifitegrast ophthalmic solution or varenicline nasal spray off-label to treat SjS-associated dry eye, but EULAR makes no recommendation for their use.
Serum eye drops are blood-derived eye drops that may be autologous (uses the patient’s own blood) or allogenic (the blood comes from a donor).26 These are compounded; a specialized pharmacy collects the patient’s blood, then clots, centrifuges, and dilutes it with sterile saline. The serum drops also contain increased concentrations of proteins, growth factors, vitamins A and C, antioxidants, and electrolytes found in natural tears.26 This is meant to mimic natural basal tears’ biochemical properties to heal the cells of the ocular surface.
Small uncontrolled studies have examined serum eye drops for SjS patients, and results are inconsistent.11 Nevertheless, ophthalmologists may use this option for patients with severe symptoms who are refractory to topical cyclosporine drops. When considering serum eye drops, individuals should consider storage needs, as they should be frozen until use (up to six months) and then refrigerated once opened for up to one week. Contamination during and after the compounding process is also possible.11
Studies have investigated the utility of other therapies—hydroxychloroquine, immunosuppressive agents, and rituximab—for SjS-related ocular dryness, but EULAR does not recommend any of them for ocular dryness alone based on available clinical data.
Fatigue and Pain
Patients with pSjS often present with general non-inflammatory joint/muscle pain and fatigue/weakness. After ruling out potential concomitant conditions (e.g., osteoarthritis, hypothyroidism, vitamin deficiencies, depression), providers should evaluate whether the patient is experiencing joint pain (arthralgia) or joint inflammation (arthritis, tenosynovitis).11 The ESSDAI score defines low articular activity level as arthralgia in the hands, wrists, ankles, and feet accompanied by morning stiffness longer than 30 minutes, always ruling out concomitant osteoarthritis.17 Objective inflammation (i.e., redness, heat, and swelling) in one or more joints is considered arthritis, and the ESSDAI score classifies arthritis severity based on the number of joints involved. Management of arthritis is covered under systemic disease treatment, and Table 3 outlines EULAR recommendations for non-arthritis musculoskeletal pain.
Table 3. EULAR-Recommended Management of SjS-Associated Musculoskeletal Pain*11
| Acute Pain | Frequent Acute Pain | Chronic, Daily Pain |
| · Acetaminophen or NSAIDs for symptomatic relief for up to 7 to 10 consecutive days
· Topical diclofenac may be effective for local pain with fewer adverse effects |
· Consider hydroxychloroquine in patients with articular pain based on its evidence for use in other SAD
· Off-label use of biologics (even as rescue therapy) is not recommended |
· Emphasize non-pharmacologic management (e.g., physical activity) before medications
· Goal is to avoid repeated use of NSAIDs or glucocorticoids · Musculoskeletal: antidepressants and anticonvulsants · Neuropathic: gabapentin, pregabalin, or amitriptyline · Opioids are not recommended |
*Providers must first rule out concomitant osteoarthritis (i.e., objective inflammation in 1 or more joints); NSAID = non-steroidal anti-inflammatory drug; SAD = systemic autoimmune diseases
Systemic Disease
EULAR defines systemic SjS as disease involvement that affects or has affected any of the organs/systems included in the ESSDAI score (i.e., all domains except biological).11 Systemic disease is linked to autoimmune dysfunction that may eventually become irreversible. Providers should limit systemic therapies to patients with active systemic disease following careful evaluation of symptom severity and organ damage. Clinicians should consider systemic therapy on an individual basis, as not all patients with active systemic disease will require it.11
EULAR makes a few general recommendations regarding systemic therapy11:
- Consider systemic therapies for most patients presenting with at least moderate activity in one clinical domain, or with a global moderate disease activity score (i.e., greater than 5).
- Therapeutic response is considered a reduction of 3 or more points in the global ESSDAI score.
- Providers should follow a sequential (or combined) use of glucocorticoids, immunosuppressive agents, and biologics to treat organ-specific systemic manifestations.
- Use glucocorticoids at the minimum dose and duration necessary to control active systemic disease.
- Use synthetic immunosuppressive agents (e.g., azathioprine, cyclophosphamide, leflunomide, methotrexate, mycophenolate) mainly as glucocorticoid-sparing agents in patients requiring long-term glucocorticoid therapy (i.e., those with severe organ impairments).
- Consider B-cell targeted therapies (e.g., belimumab, rituximab) in patients with severe, refractory systemic disease.
Evidence regarding the use of glucocorticoids for SjS is weak and studies report high rates of AEs.11 Guidelines recommend administering pulses of methylprednisolone followed by doses of 0.5 mg/kg daily or less as induction therapy in patients with severe disease and lower doses in patients with less severe disease. The goal is to withdraw glucocorticoids in patients whose SjS becomes inactive as soon as possible or at least target a maintenance dose of 5 mg daily or less with the aid of steroid-sparing immunosuppressive agents.11 Studies of immunosuppressive agents are lacking, so EULAR does not recommend one agent over another, except in the case of patient characteristics or comorbidities. Dose, route of administration, and duration of treatment are not established given the lack of clinical data, so physicians should follow similar dosing schedules to other systemic autoimmune diseases.11
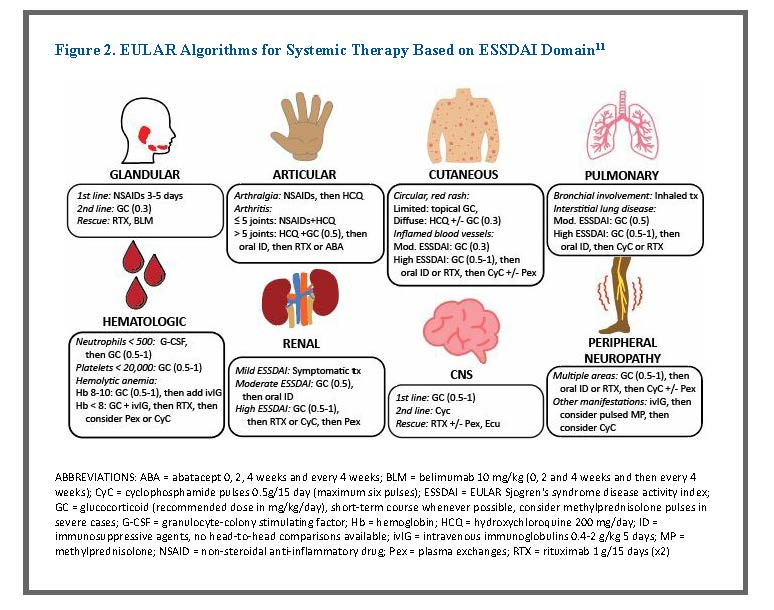
EULAR guidelines also include algorithms for each ESSDAI domain based on available trial data and the clinical experience of the individuals on the EULAR task force.11 Figure 2 summarizes these recommendations, including standard of care, second line, and third line recommendations.
Considering Comorbidities
More than 20% of people with SjS are older than 65 years, making them more likely to have pulmonary, liver, kidney, or heart-related comorbidities.19 It is especially important to consider alternative causes of sicca in older patients since many conditions and drugs produce oral and ocular dryness. Older people are nearly twice as likely to suffer from dry eyes than younger individuals.19 Older age is also associated with decreased salivary flow rate. Dry mouth is more than a bothersome symptom. Addressing dry mouth in older adults is vital because worsening oral health increases risks of malnutrition, social isolation, care dependency, and frailty that tend to affect this population.19
An estimated 45% to 80% of the older adult population reports some pain, most commonly musculoskeletal.19 Treatment plans for these patients should emphasize non-pharmacologic relief rather than medications as first-line therapy. Also, despite the lack of available evidence, experts suggest that topical NSAID formulations may be effective for local SjS-related pain in older adults with fewer AEs than oral NSAIDs.19
Treating systemic symptoms in this population also requires special considerations. Older adults are more likely to experience AEs from glucocorticoids—including blood clots, osteoporosis, and bone fractures—than younger individuals.19 Pharmacists should also consider the greater frequency of hepatic and renal impairment in older patients. For example, renal function decline and decreased folate stores may increase methotrexate-related toxicity.19 Studies suggest that disease-modifying antirheumatic drugs (including some biologics) have similar effects on younger and older patients while maintaining favorable AE profiles.19 Older people, therefore, should not be excluded from the use of these agents for systemic disease where appropriate.
Autoimmune conditions increase the risk of lymphoma, cancer stemming from the lymph nodes.33 More specifically, pSjS is the autoimmune disease associated with the highest risk of B-cell lymphoma, occurring in 5% to 10% of patients.33 This risk increases by 2.2% per year of age in this population.33 In patients with pSjS, chronic stimulation of autoimmune B cells leads to development of B-cell lymphoma. Screening for lymphoma is an important part of a comprehensive treatment plan given the increased risk. Similar to other autoimmune conditions, SjS also increases patients’ risk for atherosclerosis and coronary artery disease.22
Research Continues
Researchers continue to define new therapeutic targets and investigate new treatments for SjS. Targeting B cells appears to be the most promising therapeutic approach for this condition.18 Studies are evaluating anti-CD20 antibodies and antibodies targeting the BAFF signaling pathway to target B cells and anti-CD40 antibodies to block the crosstalk between T cells and B cells.18 So far, two agents have met their primary outcome—improvement in systemic disease activity—in pSjS clinical trials: anti-BAFF receptor antibodies and anti-CD40 antibodies.
BAFF receptors are exclusively expressed on B cells, so targeting these receptors effectively depletes B cells to blunt the autoimmune response in pSjS. Clinical trials have assessed an anti-BAFF receptor antibody, ianalumab (VAY736) in patients with pSjS with positive results.34,35 In the phase 2b study, patients experienced improved ESSDAI scores from baseline to week 24 and improvement in stimulated saliva flow rate.35 This is a promising option for a future disease-modifying pSjS treatment, and phase 3 trials are ongoing.
The interaction between CD40 and the CD40 ligand (CD40L) is important for B cell development, antibody production, and optimal T cell-dependent antibody responses. Patients with pSjS have increased expression of CD40L compared to healthy individuals, which suggests that CD40-CD40L interactions could be a practical target for pSjS treatment.18 Phase 2 studies have shown promising results for iscalimab, an anti-CD40 antibody, to treat pSjS. Patients treated with intravenous iscalimab experienced a mean ESSDAI score decrease of 5.21 points, a significant improvement over patients in the placebo group.36 Phase 3 studies of this therapy are forthcoming. A phase 2 trial of another anti-CD40L antibody is also underway.37
Additional therapeutic targets under investigation include18
- Bruton’s tyrosine kinase, an important molecule in B cell receptor signaling
- plasmacytoid dendritic cells, which secrete type 1 IFNs
- downstream type 1 and 2 IFN signaling (using Janus kinase inhibitors)
- IL-12 signaling pathway and induction of T helper 1 cells, which secrete type 2 IFNs (using ustekinumab)
PAUSE AND PONDER: How often do you encounter patients asking for help choosing artificial tear products? What could you improve about your ability to assist them?
MULTIDISCIPLINARY TEAM CARE IS IDEAL
EULAR guidelines recommend a multidisciplinary approach to SjS treatment.11 This is the second strongest recommendation included in the 2020 guidelines, with only a recommendation for patients who develop B-cell lymphoma to receive individualized treatment receiving a stronger grade. SjS’s overall prevalence in the general population is low and the condition presents differently in every patient, making it difficult for any one provider to ensure in-depth expertise in managing it. At minimum, the SjS care team should include a primary care provider (PCP), a rheumatologist, a dentist, and an ophthalmologist. Pharmacy staff should understand the roles and responsibilities of each provider to better recognize their own place on the care team.
Rheumatologist
Rheumatologists are usually the “lead” of the medical team for SjS and have the primary responsibility for managing it.38 The rheumatologist should verify the diagnosis, including looking for disease mimics and screening and monitoring for coexisting rheumatologic or autoimmune conditions. They should also screen for lymphoma risk factors and common comorbidities. They may collaborate with the patient’s PCP for comorbidity monitoring and management. Rheumatologists also provide treatment for systemic features of SjS.38
Primary Care Provider
The PCP should provide routine, comprehensive health care addressing a wide range of issues, including patients’ mental health.38 They should collaborate with the patient’s rheumatologist to establish who is responsible for overlapping areas of practice (e.g., comorbidities, immunizations, nutrition concerns). Screening for comorbidities—including cardiovascular disease, osteoporosis, sinusitis, and others—is an important task for PCPs, but they may be unaware of the increased risk of these conditions in patients with SjS. Pharmacy teams should encourage patients to advocate for themselves, and direct patients to www.sjogrensadvocate.com for advice on how to do so effectively.38
Ophthalmologist
Ophthalmologists are responsible for managing severe dry eye.38 Occasionally, the ophthalmologist is the first provider to suspect SjS and refers patients to rheumatology for general management. They perform diagnostic tests (e.g., Schirmer’s test, ocular staining score, tear breakup time) to determine the severity of SjS ocular symptoms and blood tests to screen for biologic signs of SjS. These clinicians also provide routine dry eye management, including prescription medications/drops and recommendations for OTC therapies.
Dentist
Many patients with SjS require extensive dental care exceeding the recommended checkups every 6 months for otherwise healthy individuals.38 Preventative dental visit frequency depends on patients’ level of dryness and decay. At every checkup, dentists should examine the entire oral region, including palpating (i.e., feeling with the fingers) salivary glands, face, and neck for swelling and masses.38 They should also provide dental caries prevention, screening, and treatment.
Where Pharmacy Fits In
Most often, pharmacy technicians will encounter patients with SjS at the pickup counter, so they should be prepared to refer patients to the pharmacist when appropriate. Sometimes, patients request assistance finding the eye care aisle for OTC drops. Before pointing them in the right direction, pharmacy technicians should refer patients to the pharmacist for counseling if they indicate they are new to using artificial tears (e.g., asking your opinion about product selection).
Technicians can also help patients locate products based on pharmacist recommendations and provide informational handouts about proper administration technique (see Sidebar). While cost is an important factor in therapy adherence, consider recommending name brand products rather than store brand generics whenever feasible. While the active ingredients may be consistent across proprietary and store brand products, the concentration of these components is often less than 5% of each drop.39 The amount of inactive ingredients (i.e., “filler”) differs from brand to brand.
Sidebar: Don’t Leave Patients High and Dry40,41
To provide maximum relief, patients must administer eye formulations correctly. Many patients struggle with this, especially older patients, and joint pain in SjS can make it even more difficult. Counseling patients on proper eye drop and ointment instillation is crucial to improving outcomes.
Eye Drop Administration
- Thoroughly wash hands and areas of the face around the eyes. Remove contact lenses unless the product is specifically designed for use with contact lenses. If using a suspension, shake well.
- Tilt head back, gently grasp the lower eyelid below lashes and pull away from the eye to create a pouch.
- Look up and administer a single drop into the pouch without touching the tip of the container to the eye.
- As soon as the drop is instilled, release the eyelid slowly. Close eyes gently for 3 minutes and position the head downward (gravity pulls the drop onto the ocular surface). Minimize blinking or squeezing the eyelid.
- Use a finger to gently apply pressure to the opening of the tear duct (inner corner of the eye) to prevent medication from draining through the tear duct and increase medication contact time in the eye.
- If additional ophthalmic therapy is indicated, wait 5 to 10 minutes in between. Also, wait 5 to 10 minutes before reinserting contact lenses, if applicable.
Pro-Tip: tell patients, “If you have a hard time deciphering whether you’ve successfully installed eye drops, refrigerate the solution before administration to detect the drops more easily on your eye’s surface. Do NOT use this trick with a suspension.”
Eye Ointment Administration
- Thoroughly wash hands and areas of the face around the eyes. Remove contact lenses unless the product is designed for use with contact lenses specifically.
- Tilt head back, gently grasp the lower eyelid below lashes, and pull away from the eye to create a pouch.
- Look up, and with a sweeping motion, place a strip of ointment ¼ to ½ inch wide inside the lower eyelid by gently squeezing the tube (avoid touching the tube tip to any tissue surface).
- Release the eyelid slowly and close eyes gently for 1 to 2 minutes.
- Vision may blur temporarily, so avoid activities that require good visual acuity until vision improves. Also, wait 15 minutes before reinserting contact lenses, if applicable.
Clearly, a medication expert needs to contribute to patient and provider education and oversee prescribed and OTC medications. Pharmacists can offer various clinical pearls to help patients with SjS avoid dry eyes, mouth, and skin.
Lifestyle modifications42:
- Avoid windy or drafty environments and wear sunglasses outdoors
- Use a humidifier indoors to keep the air moist
- Practice good oral hygiene (e.g., chew sugarless gum, stay well hydrated, see a dentist three times a year)
- Consciously remember to blink when working at a computer or reading extensively
- Avoid wearing eye makeup
- Consider smoking cessation and avoid smoky environments
- After showering, pat dry gently and apply an emollient to damp skin within three minutes
Separation and timing40,41:
- Separate administration of multiple eye drops by at least 5 minutes to ensure the first drop is not flushed away by the second and the second drop is not diluted by the first
- If using multiple products, use them in order of least viscous to most viscous to ensure efficacy of all treatments
- If using drops and ointment, administer drops at least 10 minutes before ointment so the ointment does not create a barrier to the drops’ absorption
- If using a suspension with another dosage form, use the suspension last because its retention time in the tear film is longer
Pharmacists and pharmacy technicians should also be aware of medications that could worsen symptoms of dryness (Table 4). Technicians should refer patients with SjS to the pharmacist when they see these at the pick-up counter. They should also stay up to date on available eye care formulations and discuss new products with the pharmacist. Pharmacists should counsel patients with SjS about which OTC products to avoid and offer to contact prescribers to recommend prescription therapy changes.
Table 4. Medications That Cause or Worsen Ocular Dryness19,25,28
| Medication/Class | Examples | Rx/OTC | Mechanism for Ocular Dryness |
| Anticholinergics | benztropine
trihexyphenidyl |
Rx | Blocking acetylcholine blurs vision and stops the signals that normally tell the eyes to produce tears |
| Antihistamines (especially first-generation) | cetirizine
chlorpheniramine diphenhydramine loratadine |
OTC
|
Dry secretions (including tears) and produce anticholinergic adverse effects |
| Beta-blockers | atenolol
metoprolol propranolol |
Rx | Cause the body to make less of a protein present in tears, and can lower pressure in the eyes, affecting the amount of water in the tears |
| Decongestants | phenylephrine
pseudoephedrine |
OTC | Decrease nasal/mucosal mucus production (including the eyes), which decreases tear production |
| Diuretics | furosemide
hydrochlorothiazide |
Rx | Help the body eliminate water and salt, which can alter tear composition |
| Hormones | estrogen/progesterone and other hormones used for contraception, infertility, or hormone replacement | Rx | Unknown |
| Isotretinoin | N/A | Rx | Lessens oil production by certain glands to treat acne, but some of those glands are in eyelids, decreasing oil in tears |
| Tricyclic antidepressants | amitriptyline
amoxapine clomipramine imipramine maprotiline |
Rx | Anticholinergic adverse effect stops the signals that normally tell the eyes to produce tears |
OTC = over-the-counter; Rx = prescription only
CONCLUSION
SjS is a complex, multifactorial condition that impacts patients’ quality of life substantially. Providing optimal care for this disease requires a multidisciplinary team, on which pharmacists and pharmacy technicians provide a link between all providers to ensure continuity of care. Recognizing patients with SjS in the pharmacy is crucial to prevent polypharmacy, ensure patients know how to use eye care formulations, assist patients in finding OTC products to address symptoms, and refer to other providers when necessary. This will improve care and outcomes for patients with SjS.
Pharmacist Post Test (for viewing only)
Pharmacists Post-test
Upon completion of this activity, pharmacists will be able to
1. DISCUSS current theories postulating how Sjogren’s syndrome develops
2. IDENTIFY biomarkers used in diagnosis and patient classification
3. INTERPRET guidelines and evidence-based medicine to use best practices to manage Sjogren’s syndrome
4. USE elements of an integrated approach to care among specialists and other pharmacists
1. Which gene(s) shows the strongest association with primary SjS?
A. STAT4
B. IRF5
C. HLA
2. Which of the following is associated with SjS pathogenesis?
A. Interferon signature
B. T-cell activating factor
C. Epstein-Barr virus
3. Which sentence describes the potential role of BAFF in primary SjS development?
A. It is an unexplored and unreliable therapeutic target for SjS treatment
B. It proves that epithelial cells are passive victims of SjS autoimmunity
C. It serves as a link between the innate and adaptive immune systems
4. Which of the following biomarkers may be more accurate than traditional clinical tests for SjS detection?
A. TSAs
B. LACTO and LIPOC-1
C. S100A8/A9
5. Which is TRUE about the ESSDAI score?
A. A 14 is the highest score possible
B. It measures disease activity in 12 domains
C. It assesses patient-reported outcomes
6. A patient consults with you about her SjS-induced dry mouth symptoms. She has been using a gel saliva substitute for a week. It works well, but she finds it annoyingly sticky and is hoping to find an alternative. She tells you her rheumatologist says she has mild gland dysfunction and acceptable saliva output. What is the best recommendation for this patient?
A. Dilute the saliva substitute with water
B. Switch to xylitol-free chewing gum
C. Talk to your rheumatologist about trying cevimeline
7. A patient is using artificial tear drops for SjS-related ocular dryness, but he complains that he must use them every 2 hours because they wear off. Which of the following is the best recommendation for this patient?
A. Switch to an artificial tear suspension containing hyaluronate
B. Switch to an artificial tear ointment containing BAK
C. Talk to your ophthalmologist about prescription therapies
8. A patient with SjS complains of visible redness, considerable heat, and ample swelling in three of his joints. He brings acetaminophen and ibuprofen to your pharmacy counter and asks which one will work better. Which of the following is the best recommendation for this patient?
A. Ibuprofen is the better choice because it is anti-inflammatory
B. A topical NSAID like diclofenac is a better choice because it is locally-acting
C. Talk to your rheumatologist about systemic hydroxychloroquine with NSAIDs
9. A patient presents to your pharmacy to buy artificial tears. She mentions that her ophthalmologist recommended that she see a rheumatologist because she thinks the patient has SjS. She doesn’t understand why that’s necessary when she can just use OTC drops to lubricate her dry eyes, and she doesn’t plan to see another provider. Which of the following is the best response?
A. You can use OTC drops as long as you choose a product with methylcellulose and no benzalkonium chloride
B. SjS affects your whole body, not just your eyes, so you may need additional treatment from a rheumatologist
C. Your ophthalmologist can prescribe prescription therapies for your dry eye symptoms, so you don’t need to see a rheumatologist
10. A patient’s neurologist prescribed propranolol for migraine prevention. He presents to your pharmacy to pick up the prescription along with a facewash for acne and artificial tear drops for SjS. What should you do?
A. Offer to contact the patient’s neurologist for an alternative migraine prevention therapy
B. Recommend a suspension, not drops, to prevent blurred vision that could worsen his migraines
C. Advise him to avoid the acne facewash as it could worsen his SjS-related dry eye symptoms
Pharmacy Technician Post Test (for viewing only)
Pharmacy Technician Post-test
Upon completion of this activity, pharmacy technicians will be able to
1. DESCRIBE Sjogren’s syndrome’s basic pathology and symptoms
2. OUTLINE prescription and non-prescription treatments used in Sjogren’s syndrome
3. IDENTIFY when to refer patients to the pharmacists for recommendations or referrals
1. Which is the most common symptom of SjS?
A. Arthralgia
B. Fatigue
C. Sicca
2. Which of the following sentences accurately describes SjS symptoms?
A. Symptoms are the same in every patient
B. Symptoms may cycle between mild and severe
C. Younger patients have worse symptoms than older patients
3. Which gene(s) shows the strongest association with primary SjS?
A. STAT4
B. IRF5
C. HLA
4. Which of the following should ALL patients with SjS-related dry mouth use?
A. Gel formulation saliva substitute
B. Prescription muscarinic agonists
C. Neutral pH sodium fluoride gel
5. Which of the following has the lowest viscosity?
A. Eye drops
B. Eye suspensions
C. Eye ointments
6. Which prescription therapy does EULAR recommend for SjS-related ocular dryness?
A. Hydroxychloroquine oral tablets
B. Cyclosporine ophthalmic solution
C. Lifitegrast ophthalmic solution
7. Which of the following is used to treat frequent acute SjS-associated articular pain?
A. Hydroxychloroquine
B. Biologics
C. Amitriptyline
8. Which of the following does EULAR recommend for patients with SjS who present with ocular dryness?
A. Use artificial tears containing methylcellulose of hyaluronate at least twice daily
B. Use artificial tears containing benzalkonium chloride at least four times daily
C. Use artificial tear ointments during the day because they last the longest in the eyes
9. A patient is picking up a pilocarpine prescription for SjS-induced dry eyes. She mentions that she has daily, throbbing pain in her back. She is also purchasing naproxen (an NSAID) that she hopes will help with the pain and OTC artificial tears for her dry eyes. Why should you refer this patient to the pharmacist?
A. Acetaminophen is a better choice for this patient’s pain
B. The patient should not use artificial tears with pilocarpine
C. This patient may require prescription treatment for her pain
10. Which of the following patients with SjS should you refer to the pharmacist?
A. A 74-year-old male purchasing topical diclofenac for local, acute pain
B. A 52-year-old female purchasing artificial tears, cevimeline, and phenylephrine
C. A 33-year-old female purchasing artificial tears and insulin for diabetes
References
Full List of References
References
1. Talal N. Sjögren's syndrome: historical overview and clinical spectrum of disease. Rheum Dis Clin North Am. 1992;18(3):507-515.
2. Jonsson R, Brokstad KA, Jonsson MV, et al. Current concepts on Sjögren's syndrome - classification criteria and biomarkers. Eur J Oral Sci. 2018;126(Suppl 1):37-48. doi:10.1111/eos.12536
3. Sjogren’s syndrome. National Institute of Dental and Craniofacial Research. Updated July 2018. Accessed June 1, 2022. https://www.nidcr.nih.gov/health-info/sjogrens-syndrome
4. Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjögren syndrome. Arch Intern Med. 2004;164(12):1275-1284. doi:10.1001/archinte.164.12.1275
5. Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part I. Arthritis Rheum. 2008;58(1):15-25. doi:10.1002/art.23177
6. Venus Williams stands up for Sjogren’s awareness. Sjogren’s Foundation. April 30, 2022. Accessed June 1, 2022. https://www.sjogrens.org/blog/2022/venus-williams-stands-up-for-sjogrens-awareness
7. Taylor T. Perseverance in pursuit: U.S.'s Boxx eyes World Cup title despite illness. June 5, 2015. Accessed June 1, 2022. https://www.si.com/soccer/2015/06/05/shannon-boxx-womens-world-cup-us-national-team
8. Ramirez CD. Carrie Ann Inaba announces leave of absence from The Talk to focus on her health. April 26, 2021. Accessed June 1, 2022. https://people.com/tv/carrie-ann-inaba-taking-leave-of-absence-from-the-talk-to-focus-on-health/
9. Zintzaras E, Voulgarelis M, Moutsopoulos HM. The risk of lymphoma development in autoimmune diseases: a metaanalysis. Arch Intern Med. 2005;165:2337-2344.
10. Lazarus, M.N.; Robinson, D.; Mak, V.; Møller, H.; Isenberg, D.A. Incidence of cancer in a cohort of patients with primary sjogren’s syndrome. Rheumatology. 2006;45:1012-1015.
11. Ramos-Casals M, Brito-Zerón P, Bombardieri S, et al. EULAR recommendations for the management of Sjögren's syndrome with topical and systemic therapies. Ann Rheum Dis. 2020;79(1):3-18. doi:10.1136/annrheumdis-2019-216114
12. Kittridge A, Routhouska SB, Korman NJ. Dermatologic manifestations of Sjögren syndrome. J Cutan Med Surg. 2011;15(1):8-14. doi:10.2310/7750.2010.09033
13. Engel GL. The clinical application of the biopsychosocial model. J Med Philos. 1981;6(2):101-123. doi:10.1093/jmp/6.2.101
14. Tzioufas AG, Voulgarelis M. Update on Sjögren's syndrome autoimmune epithelitis: from classification to increased neoplasias. Best Pract Res Clin Rheumatol. 2007;21(6):989-1010. doi:10.1016/j.berh.2007.09.001
15. Carsons SE, Patel BC. Sjogren Syndrome. In: StatPearls. Treasure Island (FL): StatPearls Publishing; November 2, 2021. https://www.ncbi.nlm.nih.gov/books/NBK431049/
16. Valtýsdóttir ST, Gudbjörnsson B, Lindqvist U, Hällgren R, Hetta J. Anxiety and depression in patients with primary Sjögren's syndrome. J Rheumatol. 2000;27(1):165-169.
17. Seror R, Bowman SJ, Brito-Zeron P, et al. EULAR Sjögren's syndrome disease activity index (ESSDAI): a user guide. RMD Open. 2015;1(1):e000022. Published 2015 Feb 20. doi:10.1136/rmdopen-2014-000022
18. Seror R, Nocturne G, Mariette X. Current and future therapies for primary Sjögren syndrome. Nat Rev Rheumatol. 2021;17(8):475-486. doi:10.1038/s41584-021-00634-x
19. Retamozo S, Baldini C, Bootsma H, et al. Therapeutic recommendations for the management of older adult patients with Sjögren's syndrome. Drugs Aging. 2021;38(4):265-284. doi:10.1007/s40266-021-00838-6
20. Jonsson R. Disease mechanisms in Sjögren's syndrome: What do we know? Scand J Immunol. 2022;95(3):e13145. doi:10.1111/sji.13145
21. Nocturne G, Mariette X. Advances in understanding the pathogenesis of primary Sjögren's syndrome. Nat Rev Rheumatol. 2013;9(9):544-556. doi:10.1038/nrrheum.2013.110
22. Skarlis C, Raftopoulou S, Mavragani CP. Sjogren's syndrome: recent updates. J Clin Med. 2022;11(2):399. doi:10.3390/jcm11020399
23. Cafaro G, Croia C, Argyropoulou OD, et al. One year in review 2019: Sjögren's syndrome. Clin Exp Rheumatol. 2019;37 Suppl 118(3):3-15.
24. Evoxac [prescribing information]. Daiichi Pharmaceutical Co.; 2006. https://www.accessdata.fda.gov/drugsatfda_docs/label/2006/020989s008lbl.pdf
25. Paulsen AJ, Cruickshanks KJ, Fischer ME, et al. Dry eye in the beaver dam offspring study: prevalence, risk factors, and health-related quality of life. Am J Ophthalmol. 2014;157(4):799-806. doi:10.1016/j.ajo.2013.12.023
26. Dang VT, Hoyle B. Autologous serum tears: an overlooked treatment for dry eye. Modern Optometry. July 2020. Accessed June 1, 2022. https://modernod.com/articles/2020-july-aug/autologous-serum-tears-an-overlooked-treatment-for-dry-eye?
27. Pucker AD, Ng SM, Nichols JJ. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst Rev. 2016;2:CD009729. doi:10.1002/14651858.CD009729.pub2
28. Messmer EM. The pathophysiology, diagnosis, and treatment of dry eye disease. Dtsch Arztebl Int. 2015;112(5):71-82. doi:10.3238/arztebl.2015.0071
29. Benzalkonium chloride (BAK). Not a Dry Eye. Accessed June 13, 2022. https://www.notadryeye.org/all-about-dry-eye-syndrome/treatments-for-dry-eye-syndrome-and-related-conditions/lubricating-eye-drops/glaucoma-eyedrops-a-fresh-look-at-preservatives/
30. CFR—Code of Federal Regulations Title 21. U.S. Food and Drug Administration. Updated March 28, 2022. Accessed June 13, 2022. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfCFR/CFRSearch.cfm?CFRPart=349
31. Horton M, Horton M, Reinhard E. Master the maze of artificial tears. Review of Optometry. November 20, 2018. Accessed June 13, 2022. https://www.reviewofoptometry.com/article/master-the-maze-of-artificial-tears
32. Gupta PK, Asbell P, Sheppard J. Current and future pharmacological therapies for the management of dry eye. Eye Contact Lens. 2020;46 Suppl 2:S64-S69. doi:10.1097/ICL.0000000000000666
33. Nocturne G, Pontarini E, Bombardieri M, Mariette X. Lymphomas complicating primary Sjögren's syndrome: from autoimmunity to lymphoma. Rheumatology (Oxford). 2019;60(8):3513-3521. doi:10.1093/rheumatology/kez052
34. Dörner T, Posch MG, Li Y, et al. Treatment of primary Sjögren's syndrome with ianalumab (VAY736) targeting B cells by BAFF receptor blockade coupled with enhanced, antibody-dependent cellular cytotoxicity. Ann Rheum Dis. 2019;78(5):641-647. doi:10.1136/annrheumdis-2018-214720
35. Dörner T, Bowman SJ, Fox R, et al. Ianalumab (VAY736), a dual mode of action biologic combining BAFF receptor inhibition with B cell depletion, reaches primary endpoint for treatment of primary Sjogren’s syndrome [abstract OP0302]. Ann Rheum Dis. 79(Suppl 1);187-188 (2020).
36. Fisher BA, Szanto A, Ng WF, et al. Assessment of the anti-CD40 antibody iscalimab in patients with primary Sjögren's syndrome: a multicentre, randomised, double-blind, placebo-controlled, proof-of-concept study. Lancet Rheumatol. 2(3);e142-e152 (2020).
37. Safety, tolerability, pharmacokinetics, and therapeutic efficacy of SAR441344 in primary Sjögren's syndrome (pSjS) (phaethuSA). ClinicalTrials.gov identifier: NCT04572841. Updated May 12, 2022. Accessed June 1, 2022. https://www.clinicaltrials.gov/ct2/show/NCT04572841
38. For the newly diagnosed. Sjogren’s Advocate. Updated June 10, 2022. Accessed June 13, 2022. https://www.sjogrensadvocate.com/newly-diagnosed
39. So many choices…what drops are best for my dry eyes? Summit Eye Center. March 5, 2019. Accessed June 13, 2022. https://www.summiteyekc.com/blog/eye-drop-overload-at-the-pharmacy
40. Evans K, Madden L. Recommended dry eye treatments in community pharmacy. The Pharmaceutical Journal. August 2, 2016. Accessed June 13, 2022. https://pharmaceutical-journal.com/article/ld/recommending-dry-eye-treatments-in-community-pharmacy
41. Fiscella RG, Jensen MK. Ophthalmic disorders. In: Krinsky DL, Ferreri SP, Hemstreet BA, Hume AL, Newton GD, Rollins CJ, Tietze KJ, eds. Handbook of Nonprescription Drugs. 19th ed. Washington, DC: APhA Publications;2018:545-566.
42. Wick JY. Sjogren’s syndrome: dry as a desert. Pharmacy Times. February 18, 2014. Accessed June 13, 2022. https://www.pharmacytimes.com/view/sjogrens-syndrome-dry-as-a-desert