INTRODUCTION
Human immunodeficiency virus (HIV) attacks the body’s immune system by destroying CD4 helper T cells. These cells are required for adaptive immunity (acquired immunity that adapts to real-time pathogen exposure), as they stimulate key players in the unimpaired immune response. Once patients are infected with HIV, starting potent combination antiretroviral therapy immediately can decrease morbidity and extend life. If the virus remains unchecked and progresses, patients develop high viral loads (the amount of virus in an infected person’s blood expressed as the number of viral particles/mL of blood) and slowly deplete their CD4 helper T cells, elevating risk for opportunistic infections (e.g., pneumocystis pneumonia, bacterial infections such as pneumococcal disease, disseminated mycobacterium avium infections).1
While HIV remains a persistent problem in the United States (U.S.) across all age groups, young people are disproportionately affected. A 2018 report stated 15,820 adults and adolescents diagnosed with HIV died in the U.S. and its six dependent areas.2 Several groups have tracked HIV infections in youths and presented startling statistics. According to the Centers for Disease Control and Prevention (CDC), individuals aged 13 to 24 years accounted for 21% of all new HIV diagnoses in 2018.2 Even more concerning, experts estimate that more than 40% of HIV-infected youth are undiagnosed.3 Although public health officials and healthcare systems have made efforts to improve access to care, recent data suggests that effective HIV prevention and treatment are not reaching those who could most benefit from them (e.g., men who have sex with men [MSM], transgender persons, Black and Hispanic/Latinx people).4
HIV infection is most likely to occur through male-to-male sexual contact (81%), heterosexual contact (10%), and injection drug use (5%).5 Data from the Youth Risk Behavior Survey shows that high school students engage in behaviors that increase risk of acquiring HIV5:
- Low rates of condom use: 45.7% report not using a condom during their last sexual encounter
- Low perception of risk: 90.6% of sexually active students report never being tested for HIV
- Substance use/abuse and sex: 21.2% report drinking alcohol or using drugs before last sexual intercourse
- Multiple sexual partners: 8.6% report sexual intercourse with four or more partners
- High rates of sexually transmitted infections (STIs): about half of all reported STIs are in those aged 15 to 24 years
Sexual exploration is a normal part of adolescent development and sexual activity can be both unplanned and unwanted. In 2011-2013, 47% and 44% of 15- to 19-year-old males and females, respectively, had engaged in sexual intercourse.6 The onset of sexual activity during adolescence coupled with a propensity for substance use experimentation and low rates of condom use increase adolescents’ vulnerability to infection with HIV and other STIs.5 Therefore, sexual education including material on HIV and acquired immunodeficiency syndrome (AIDS) is crucial to prevent HIV infection among adolescents and young adults.
HIV’s greater risk and prevalence in adolescents requires focused clinical attention. Early HIV diagnosis and treatment in adolescence and young adulthood reduces risk for HIV transmission and increases the likelihood of a functional lifestyle similar to that of non-infected individuals. There are many HIV prevention methods, including
- pre-exposure prophylaxis (PrEP) and post-exposure prophylaxis (PEP) to prevent HIV infection
- avoiding risky behaviors that can result in HIV exposure/infection
- testing and treatment to reduce mother-to-child HIV transmission
National U.S. data shows that from 2012 to 2018, among commercially insured individuals at high risk for HIV/AIDS, 13% of patients aged older than 35, 9.6% of individuals aged 25 to 34, but only 2.7% of patients aged 16 to 24 had received PrEP. 2 The low prescribing rate among youths may be related to healthcare providers’ lack of knowledge about PrEP or discomfort with prescribing PrEP.2 Thus, healthcare providers who care for adolescents and young adults need education on discussing sexual health with pointed discussion about STI and HIV prevention and recommending or prescribing PrEP when appropriate.5
PRE-EXPOSURE PROPHYLAXIS
Taking antiviral medications to reduce the risk of acquiring HIV in the event of exposure is referred to as PrEP. Studies have shown PrEP is highly effective when used consistently (i.e., taken as directed) before HIV exposure during sex or injection drug use.7,8 Specifically, the CDC indicates PrEP can reduce the risk of contracting HIV from sex by about 99% and from injecting drugs by 74% to 84%.7
The most updated “Preexposure Prophylaxis for the Prevention of HIV Infection in the U.S.” clinical practice guidelines state that clinicians should offer PrEP to adolescents who weigh more than 35 kg (77 lbs) who fit the following criteria9:
- HIV negative, but participate in sexual behaviors that put them at risk for HIV infection (e.g., recent STI, sex with an HIV-positive partner, inconsistent condom use)
- HIV negative, but inject drugs
The guidelines also define clinical eligibility by the potential medication regimen9:
- All PrEP regimens (oral and injectable)
- Negative HIV test result within the last 7 days
- No signs/symptoms of acute HIV infection
- No contraindicated medications or conditions
- Oral daily regimens only (not injectable cabotegravir)
- Negative hepatitis B virus infection
- Adequate renal function (discussed below)
PAUSE & PONDER: How can you identify patients in your practice who may benefit from PrEP?
Oral PrEP Regimens
Two emtricitabine-based oral regimens are U.S. Food and Drug Administration (FDA) approved for HIV PrEP. The first to be approved was emtricitabine/tenofovir disoproxil fumarate (DF; brand name Truvada) in 2012.10 The FDA expanded the indication for HIV PrEP to at-risk adolescents (age 12 years and older) and adults weighing at least 35 kg in 2018 based on the Adolescent Medicine Trials Network for HIV/AIDS Interventions 113 (Project PrEPare) study. This study identified the safety and feasibility of antiretroviral PrEP for adolescents aged 15 to 17 years in the U.S.11 For HIV prevention, patients take one tablet daily containing 200 mg emtricitabine and 300 mg tenofovir DF.10 Individuals must have a creatinine clearance (CrCl) of 30 mL/min or greater to take Truvada or its generic.10
Preliminary evidence also suggests adult MSM who have infrequent sexual activity can take 2 doses of emtricitabine/tenofovir DF on an “on-demand” basis. Although not FDA approved for this indication, CDC guidelines recommend patients take 2 tablets (total dose emtricitabine 400 mg/tenofovir DF 600 mg) two to 24 hours prior to sexual activity, then one tablet each day for the next 2 days (i.e., 24 and 48 hours after initial dosing). In cases of continued sexual activity, patients should continue taking one tablet daily until 48 hours after the last sexual event.9 This dosing may be ineffective for other patient populations, as drug concentrations in colorectal tissues allows for more missed doses than vaginal concentrations.9
In 2019, the FDA approved a second oral combination for HIV PrEP: emtricitabine 200 mg and tenofovir alafenamide (AF) 25 mg (brand name Descovy) for at-risk adolescents (age 12 years and older) and adults weighing at least 35 kg.12,13 This indication excludes women who have receptive vaginal sex because clinical trials did not assess this patient population. Individuals must have a CrCl of 60 mL/min or greater to take Descovy.13
Both oral PrEP medication regimens offer the same level of protection (for indications evaluated), are dosed once daily, and have many similar adverse effects. However, emtricitabine-tenofovir DF has a higher risk of acute kidney injury and bone mineral density decline due to the tenofovir DF component.14 Serious adverse events from both medications are uncommon. Table 1 lists common and severe adverse effects associated with oral PrEP regimens. Although hepatotoxicity monitoring is not routinely recommended, pharmacists should advise patients to contact a provider if they experience signs and symptoms of liver dysfunction, including decreased appetite, nausea, stomach pain, yellowing of the skin or eyes, darkened urine, and light-colored stool.9 Both oral regimens include a Boxed Warning in their labeling for the possibility of severe acute exacerbations of hepatitis B and the need to use them in individuals confirmed to be HIV-negative immediately prior to initiating and at least every 3 months during use.10,13
Table 1. Adverse Effects Associated with Available Oral PrEP Medications9,10,13
| Common |
| Emtricitabine/tenofovir DF |
Headache, abdominal pain, and mild weight loss |
| Emtricitabine/tenofovir AF |
Diarrhea |
| Severe (both regimens) |
| Adverse Effect |
Monitoring/Counseling |
| Acute kidney injury/failure or worsening of chronic renal failure |
Evaluate kidney function before treatment (SCr, CrCl, eGFR) and monitor for signs and symptoms of kidney dysfunction (e.g., significantly reduced urine output) |
| Lactic acidosis |
Monitor for clinical symptoms (physical discomfort, rapid breathing or problems breathing, muscle pain, and confusion), no routine labs suggested |
| Bone issues associated with decreased BMD (e.g., pain, softening, thinning, fracture risk) |
Generally asymptomatic; refer to prescriber for fractures, pain, or other concerning bone-related symptoms. Recommend healthy diet with more vegetables and food rich in calcium, vitamin D and K. If needed, over the counter calcium supplement is recommended. |
| Abbreviations: AF, alafenamide; BMD, bone mineral density; CrCl, creatinine clearance; DF, disoproxil fumarate; eGFR, estimated glomerular filtration rate; SCr, serum creatinine |
Injectable PrEP
In December 2021, the FDA approved the first injectable therapy for HIV PrEP—cabotegravir extended-release injectable suspension (brand name Apretude)—for adolescents (12 years and older) and adults at risk of sexually-transmitted HIV infection who weigh at least 35 kg.15 The CDC released their HIV PrEP guidelines just prior to injectable cabotegravir’s approval, which may lead to confusion.9 Injectable cabotegravir is approved for adolescents and adults weighing at least 35 kg, but the guidelines only recommended it for adult persons.9,15 Also, injectable cabotegravir is approved to prevent sexually-acquired HIV, but CDC guidelines state that providers should evaluate people at risk due to injection drug use to see if they also are indicated for PrEP due to sexual activity and therefore eligible for injectable cabotegravir.9,15
A provider administers two loading doses of cabotegravir 600 mg intramuscularly one month apart, followed by 600 mg intramuscularly every two months.15 There is a 7-day grace period for dosing. If patients are unable or do not receive their scheduled dose within seven days of when it is due, providers should consult the product information to assess if the patient remains a good candidate and restart dosing as recommended. If there is concern that the patient may not tolerate intramuscular cabotegravir, providers may opt to prescribe oral cabotegravir 30 mg daily as a trial for four weeks.15 If patients take the oral lead-in, the first injection should be scheduled within three days of the last oral dose and the second injection should still occur one month after the first.15
The most common adverse effects of extended-release cabotegravir are injection site reactions (especially after the first few doses), fever, gastrointestinal upset (e.g., diarrhea, nausea, abdominal pain, decreased appetite), headache, myalgia, rash, fatigue, and sleep-related issues.9,15 Warnings and precautions for this medication include depressive disorders, hepatotoxicity, and hypersensitivity reactions.15 Cabotegravir’s labeling includes a Boxed Warning stating patients must have a confirmed negative HIV test before starting the drug and before each injection, as people with undiagnosed HIV who took cabotegravir have developed drug-resistant HIV variants.15 People who become infected with HIV while taking cabotegravir must stop the injections and switch to a complete HIV treatment regimen.15 It is also important to counsel patients that the drug will remain at low concentrations in their system for a long time (median times 44 and 67 weeks for men and women, respectively) even after discontinuing the drug.9 This low drug concentration could increase the risk of resistant virus if the patient is to become infected with HIV in that period.
The FDA’s approval of a long-acting injectable for PrEP is significant progress toward ending the HIV epidemic, as it is the first therapy to prevent HIV that is not a daily oral pill. For PrEP to be effective, it requires a high level of patient adherence, but it is challenging for many patients to remember a daily medication.15 Cabotegravir extended-release injections every two months will be crucial in increasing PrEP adherence in high-risk individuals.15
Ending the HIV epidemic requires adherence to published HIV testing recommendations, sexual health assessments, and STI screening and appropriate prevention education.2 Clinicians—including pharmacists—should be aware of the need for monitoring and follow up with HIV PrEP, listed in Figure 1. Pharmacists, as the most accessible healthcare professionals, can provide and reinforce education on sexual health (e.g., STI and HIV prevention) and recommend PrEP to those who may benefit. They should also encourage that patients combine different prophylaxis methods (e.g., condoms with PrEP) to improve protection based on CDC guidelines.9
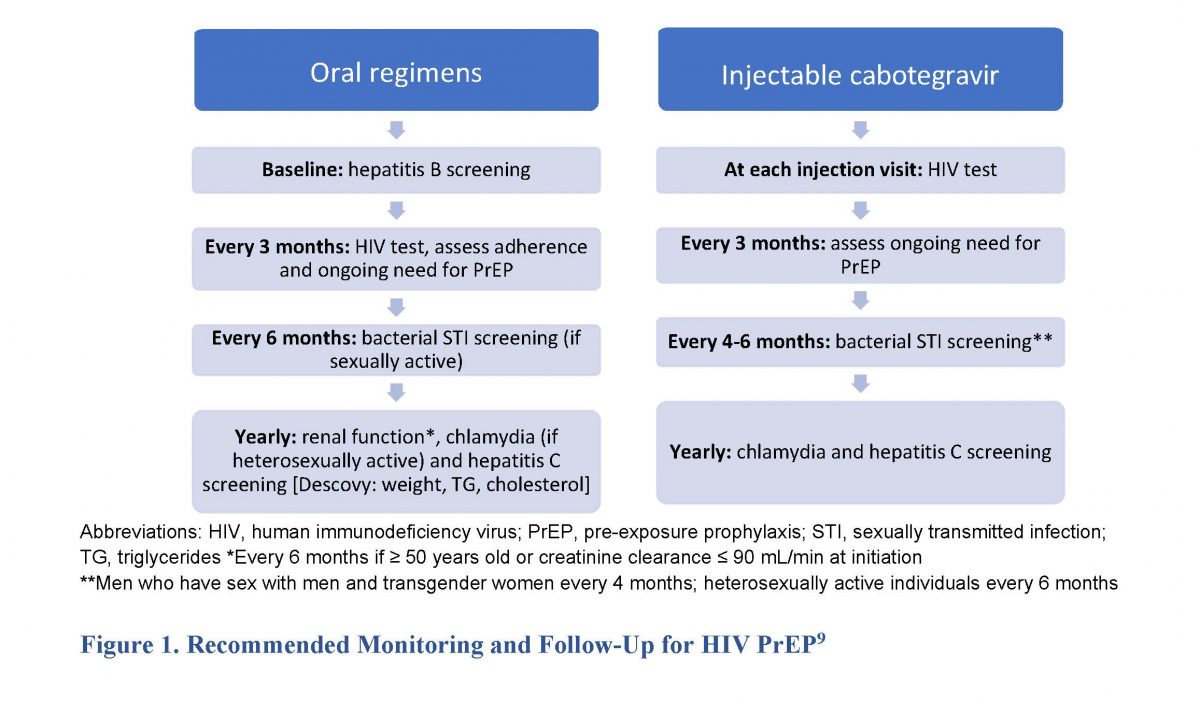
Adolescents and young adults face many barriers that lower adherence rates, including lack of awareness of HIV risk, lack of parental understanding or support, safety and adverse effect concerns, and high cost. Pharmacists and pharmacy technicians have various responsibilities to combat these barriers. Pharmacists should help patients and caregivers understand the importance of taking PrEP medications as prescribed. Adherence education can include individualized counseling on medication use, adverse effect monitoring and management, reminder text messages, and computer- and phone-based support. Pharmacy staff can also help make these medications more affordable by working with other healthcare providers and insurance companies to access patient assistance programs and navigate prior authorization (see Tech Talk SIDEBAR).16
Tech Talk: Helping Patients Afford PEP and PrEP17-21
Most insurance companies (including Medicaid) are required to cover a PrEP option free of cost. Patients requiring PEP after a sexual assault may also be qualified for full or partial reimbursement. For example, New York state Medicaid will pay for PEP medications for non-occupational health exposures, including sexual assault. The CDC provides location-specific resources to help patients: https://www.cdc.gov/hiv/basics/pep/paying-for-pep.html. After a potential HIV exposure at work, patients usually receive workers’ compensation or their workplace health insurance pays for PEP. If patients cannot obtain insurance coverage, pharmacy technicians can collaborate with healthcare providers to prepare applications for PEP or PrEP through patient assistance programs.
Patients in need of financial assistance—including those without insurance—may be eligible to receive free or low-cost PEP or PrEP medications. Manufacturers offer patient assistance programs to provide low-cost or free medications to people with low incomes. Companies have variable criteria that patients must meet to be eligible for enrollment. Applying to these programs take about five to ten minutes, and pharmacy staff should encourage prompt completion to avoid treatment delays. Patients covered under Medicaid or Medicare do not qualify for patient assistance programs or cost-sharing assistance programs.
Generic versions of emtricitabine-tenofovir DF are less expensive than brand name products and are not usually available through company patient assistance programs or cost-sharing assistance programs. However, patients may qualify for support from the Patient Assistance Foundation, Needy Meds, and/or state PrEP assistance programs. Programs like Gilead's Advancing Access Form and NASTAD’s Patient Assistance Tool list step-by-step instructions on the forms themselves. Many brand name medications are covered by their respective pharmaceutical companies. For more information on patient assistance programs for HIV PrEP medications, please refer to https://nastad.org/prep-access/prep-assistance-programs.
All medications approved for HIV PrEP are available by prescription only. Technicians who recognize these drugs can ensure the pharmacy dispenses and packages them appropriately (in their original packages to reduce the risk of contaminating the contents) and encourage adolescents and young adults to consult with the pharmacist as necessary (e.g., if they have questions, or would like education).
PrEP medications require high adherence rates from patients. Technicians might identify patients’ difficulties with adherence during a patient’s medication reconciliation in the hospital or while speaking with a patient in the community pharmacy. Red flags include late refills or failure to pick up a refill, or presenting with prescription vials that have pills remaining when they should have already taken them all.
PAUSE AND PONDER: How can pharmacy technicians help patients receive free or low-cost PEP and PrEP medications?
POST-EXPOSURE PROPHYLAXIS
Providers prescribe PEP to HIV-negative patients to prevent HIV infection after a possible exposure. After exposure to HIV, it takes two to four weeks for the virus to establish itself permanently in the body. After exposure, the body's immune system has a small window during which the viral load is small enough to be eradicated. Successful HIV PEP kills the virus before it can establish residency in the patient’s body.22 Patients must start a potent combination of HIV antiretrovirals immediately—ideally within 2 hours but no later than 72 hours after exposure.22 Every hour matters so the sooner the patient starts PEP, the lower the chance of becoming HIV infected. PEP requires a prescription from a healthcare provider and is highly effective (exceeding 80%) in preventing HIV.23
PEP is only for emergency situations and should not take the place of other regular HIV prevention methods. Pharmacy staff should advise patients who frequently require PEP to talk to their provider to see if they qualify for PrEP. Some situations requiring HIV PEP include unplanned and/or unprotected sex or sexual assault, workplace exposures (e.g., a patient’s blood accidentally splashes into a healthcare worker’s eye), or needlestick injury with a contaminated needle.24
Antiretroviral therapy for PEP involves a combination of three antiretroviral medications. According to the CDC, eligible patients should receive 28 days of PEP therapy.25 Table 2 lists the preferred antiretroviral regimens for PEP. Clinicians and patients with additional concerns or questions regarding optimal therapy options can call the National Clinical Consultations Center PEPline at (888) 448-4911.
Table 2. HIV PEP Recommendations Based on Renal Function25, 26, 27
| Adolescents and young adults aged ≥ 13 years with: |
Medication |
| Normal renal function
(CrCl ≥ 60 mL/min) |
1) emtricitabine 200 mg/tenofovir DF 300 mg once daily with
2) raltegravir 400 mg twice daily or dolutegravir 50 mg once daily* |
| Renal dysfunction
(CrCl ≤ 59 mL/min) |
1) zidovudine: Renally adjusted doses are based on oral doses of 160 mg/m2/dose every 8 hours and IV dose of 120 mg/m2/dose every 6 hours.
GFR ≥10 mL/minute/1.73 m2 and continuous renal replacement therapy: No dosage adjustment required
GFR <10 mL/minute/1.73 m2, intermittent hemodialysis, and peritoneal dialysis: Administer 50% of dose every 8 hours
and
2) lamivudine: <25 kg: There are no dosage adjustments provided in the manufacturer’s labeling; consider reducing the dose or increasing the dosing interval; use with caution and monitor closely.
≥25 kg:
CrCl ≥50 mL/minute: No adjustment necessary.
CrCl 30 to 49 mL/minute: 150 mg once daily.
CrCl 15 to 29 mL/minute: 150 mg first dose, then 100 mg once daily.
CrCl 5 to 14 mL/minute: 150 mg first dose, then 50 mg once daily.
CrCl <5 mL/minute: 50 mg first dose, then 25 mg once daily.
No additional dosing is required after routine (4 hour) hemodialysis or peritoneal dialysis.
with
3) raltegravir 400 mg twice daily or dolutegravir 50 mg once daily* |
*Dosing is the same for both normal renal function and renal dysfunction
CrCl, creatinine clearance; DF, disoproxil fumarate
PAUSE & PONDER: What are the most important counseling points for pharmacists to relay to patients about PEP and PrEP?
Headache, gastrointestinal effects (e.g., nausea, diarrhea, vomiting), and fatigue are common adverse effects of PEP regimens. In addition, clinicians should note some medication-specific adverse effects and contraindications28:
- Tenofovir is contraindicated in patients with renal dysfunction (≤ 30 mL/min for DF formulation, ≤ 60 mL/min with AF formulation)
- Emtricitabine can cause a hyperpigmented rash or skin discoloration
- Raltegravir and dolutegravir should be administered either two hours before or six hours after cation-containing products (e.g., calcium, magnesium, iron, multivitamins) because they can reduce the medications’ absorption (it is important to ask patients about these specifically, as they can be obtained over-the-counter)
- Zidovudine can cause anemia and neutropenia
- Lamivudine should not be administered with emtricitabine
- Tenofovir, emtricitabine, and lamivudine can be used in patients infected with hepatitis B, but patients will need liver function monitoring upon discontinuation because withdrawal may cause or exacerbate acute hepatitis
Patients respond differently to medications and not everybody experiences adverse effects. If patients develop life-threatening adverse effects, they need emergency attention. Subsequently, primary prescribers will need to adjust treatment plans as needed.
Pharmacists must emphasize the importance of patients taking PEP medications as prescribed and remind patients picking up these prescriptions to follow up with their providers. Further, counseling should include that these medications should be taken at the same time every day to optimize effectiveness. It is also important to counsel patients taking medications for PEP, that if they miss any doses, the likelihood of becoming infected with HIV is greater.22 Technicians should be sure to ask patients who receive PEP if they would like to talk to the pharmacist. One possible communication could be, “This is important medication, and the pharmacist may be able to answer questions you don’t even know you have!” Prompts like this can provide patient privacy while encouraging them to spend some time with the pharmacist.
Prescribers monitor patients on PEP at baseline and during the months following exposure. It is important to reinforce that patients require testing for HIV after they complete the medication regimen to ensure it was effective. Table 3 describes the CDC-recommended laboratory monitoring schedule for HIV PEP.28
Table 3. HIV PEP Required Laboratory Monitoring28
| Monitoring |
Reason(s) |
Time Period |
| HIV antigen/antibody |
Ensure the patient does not already have HIV (ineligible for PEP) and make sure PEP is effective after use |
Baseline, 4-6 weeks after exposure, and at 3 months after exposure* |
| Hepatitis B and C |
Pre-existing hepatitis infection may change therapy recommendations or monitoring |
Baseline |
| Serum creatinine |
Evaluate baseline kidney function for initial therapy choice and determining correct dosing if significant impairment exists and ensure that PEP is not causing kidney injury |
Baseline and 4-6 weeks after exposure if receiving emtricitabine/tenofovir and integrase-based regimen (e.g., bictegravir, cabotegravir, dolutegravir, elvitegravir, raltegravir) |
| Alanine transaminase and aspartate aminotransferase |
Liver function could affect therapy decisions, especially if baseline hepatitis exists |
Baseline and 4-6 weeks after exposure if receiving emtricitabine/tenofovir with an integrase inhibitor (e.g., bictegravir, cabotegravir, dolutegravir, elvitegravir, raltegravir) |
| Pregnancy test |
Pregnancy status should be incorporated into therapy decisions |
Baseline and 4-6 weeks after exposure |
| Bacterial STIs |
Identify any potential co-infections and ensure optimal, prompt therapy |
Baseline (if not done at baseline or if symptomatic, complete at 4-to-6-week follow-up) |
*additional testing at 6 months indicated if Hepatitis C acquired during exposure
HIV-RELATED STIGMA
HIV remains a highly stigmatized condition in both adults and adolescents. HIV stigma is discrimination based on negative attitudes and beliefs about the disease and the people who have it. Due to HIV’s long-standing history of prejudice and labeling, patients may have feelings of shame, fear of disclosure, isolation, and despair. This discourages individuals living with HIV from accessing treatment or staying in care, often affecting their health. Additionally, state laws concerning minors’ rights to give informed consent to receive HIV diagnosis or treatment vary across the U.S. Mental and behavioral health is often neglected, which can increase the burden on people living with HIV infection.
It is important that the healthcare team recognizes and actively addresses this stigma using interventions that ensure the well-being of affected patients. The CDC lays out two guidelines to help address the issues of problematic language and minors’ ability to consent:
CONCLUSION
Adolescents and young adults who participate in high-risk behaviors (e.g., unprotected sex, injectable drug use) are at increased risk for contracting HIV. HIV PrEP and PEP are important medical advances that decrease the risk of HIV infection for uninfected patients. CDC guidelines provide up-to-date recommendations on the medications used for PrEP and PEP. Pharmacists who dispense these medications should be prepared to educate and counsel adolescent and young adult patients at risk of HIV on the appropriate use of PrEP and PEP. Pharmacy technicians can provide significant assistance to patients obtaining HIV PrEP and PEP to ensure these medications are affordable. They can also be vigilant to refer patients obtaining these prescriptions to the pharmacist for important counseling and education.


